Your tooth enamel might look like a solid shield. However, biologically, it is a dynamic battlefield in constant flux. Every time you eat or drink, acids strip minerals away from your teeth. Saliva rushes in to replace them. In this microscopic tug-of-war, the importance of fluoride for dental health cannot be overstated. It is the deciding factor between a tooth that crumbles under bacterial attack and one that remains resilient for a lifetime.
Table of Contents
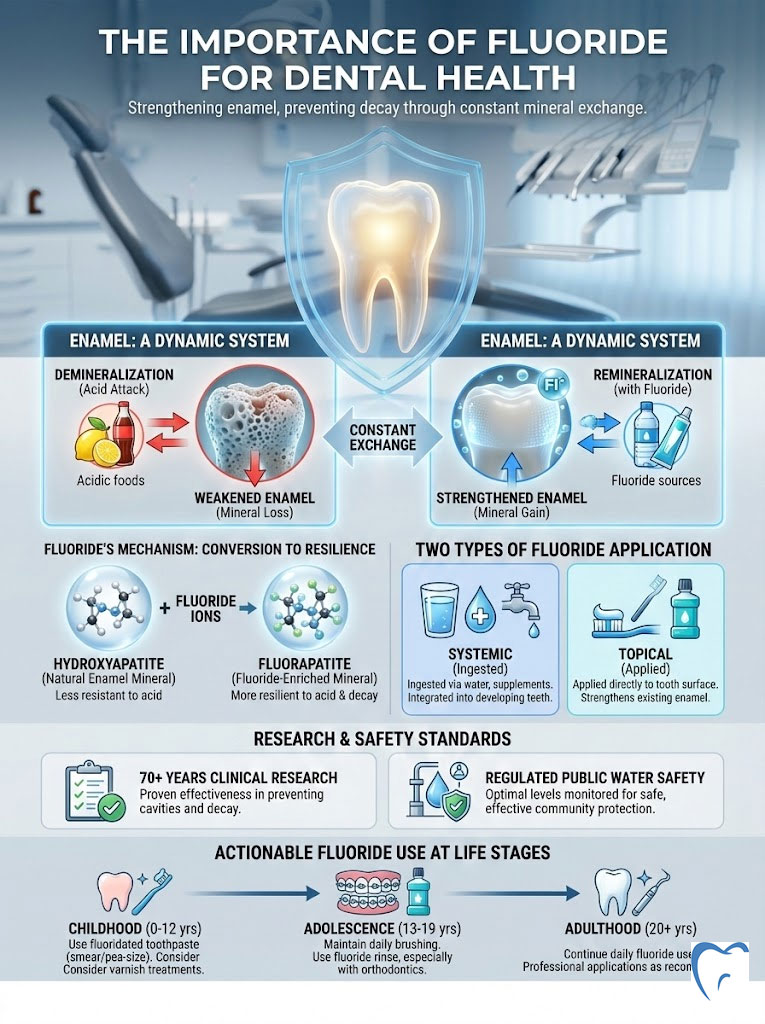
As a specialist in preventive dentistry, I often hear conflicting information from patients regarding fluoride. Some view it as a miracle mineral. Others harbor concerns about safety or toxicity. The reality is grounded in over 70 years of clinical research. Fluoride is nature’s cavity fighter. It doesn’t just clean teeth. It actively rebuilds the crystalline structure of enamel through a chemical process called remineralization. Without it, our teeth are significantly more vulnerable to the modern, sugar-rich diet that defines the American lifestyle.
This comprehensive guide will examine the molecular mechanisms of how fluoride works. We will analyze the differences between systemic and topical applications. We will also review the safety standards that guide our public water systems. We will look at the evidence. We will strip away the myths. Finally, we will provide you with actionable advice for every stage of life.
Quick Answer: Why is Fluoride Essential?
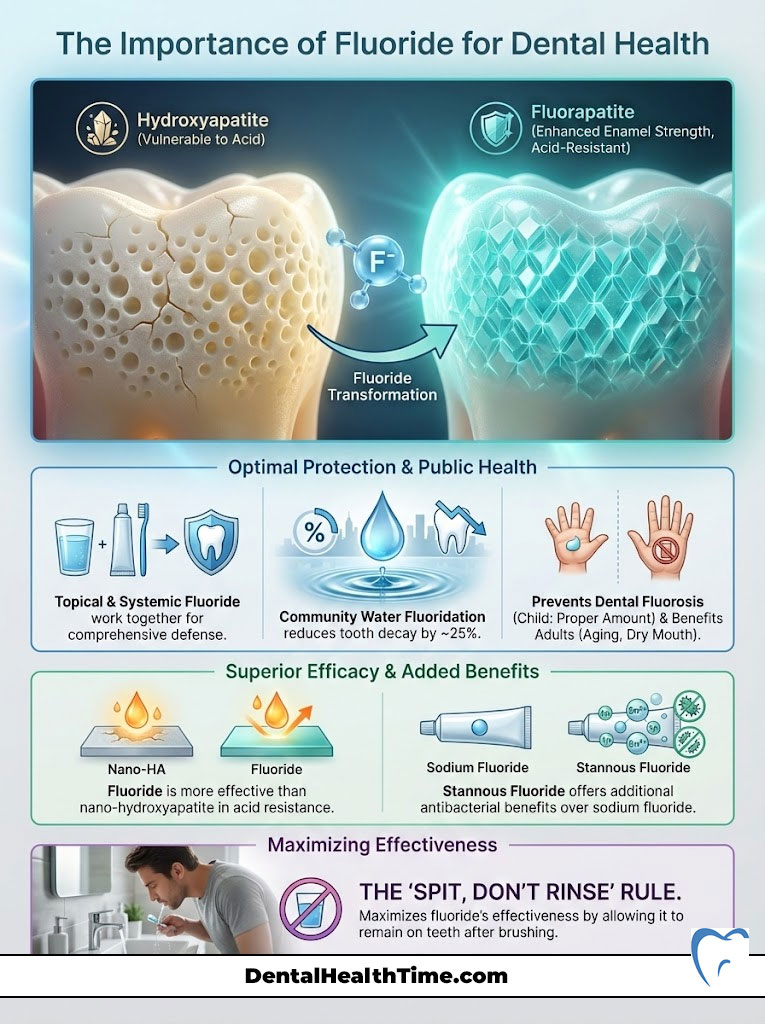
Fluoride is a naturally occurring mineral that prevents tooth decay. It works by making the outer surface of your teeth (enamel) more resistant to acid attacks from plaque bacteria and sugars in the mouth. It reverses early decay through remineralization and disrupts bacterial enzyme activity. Clinical consensus confirms that the importance of fluoride for dental health lies in its ability to convert standard tooth mineral (hydroxyapatite) into a harder, more acid-resistant form (fluorapatite).
Key Statistics: The Impact of Fluoride
- 25% Reduction: Community water fluoridation reduces tooth decay by 25% in children and adults according to the CDC.
- $20 Savings: For every $1 invested in water fluoridation, society saves approximately $20 in dental treatment costs.
- 0.7 mg/L: The optimal concentration of fluoride in drinking water recommended by the U.S. Public Health Service.
- pH 4.5: Fluoridated enamel can withstand acidity down to a pH of 4.5. Natural enamel dissolves at pH 5.5.
- $6.5 Billion: Estimated annual savings in the U.S. healthcare system due to fluoridation efforts.
- 74% Access: Approximately 74% of the U.S. population on community water systems receives fluoridated water.
The Science of Enamel: Demineralization vs. Remineralization
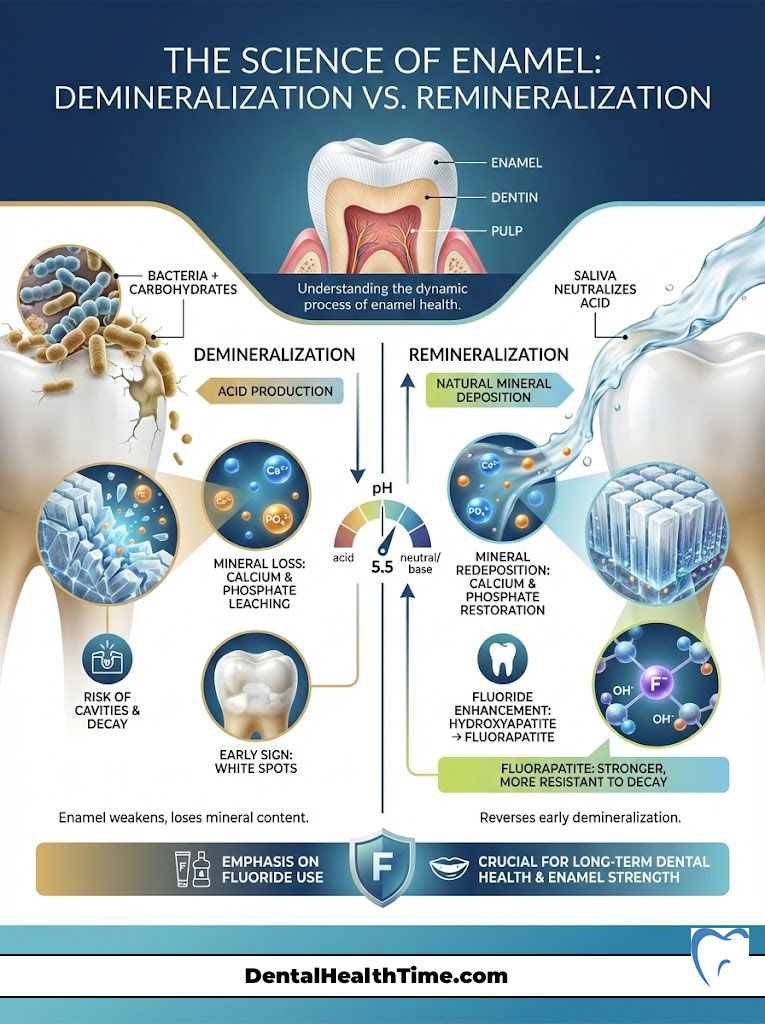
To truly grasp the benefits of fluoride for teeth, we must first understand the structure of the tooth itself. Enamel is the hardest substance in the human body. It is composed primarily of a mineral called hydroxyapatite. While it is incredibly strong, hydroxyapatite has a significant weakness: acid.
The Tug-of-War in Your Mouth
Your mouth is an ecosystem populated by billions of bacteria. When you consume carbohydrates, bacteria like Streptococcus mutans metabolize these sugars. They produce lactic acid as a byproduct. This acid lowers the pH level in your mouth. When the pH drops below a critical point (around 5.5), the calcium and phosphate ions that make up your enamel begin to dissolve. They leach out into the saliva. This process is known as demineralization.
If this process continues unchecked, the enamel structure collapses. This results in a cavity (caries). However, saliva acts as a natural buffer. It neutralizes the acid. It redeposits calcium and phosphate back into the enamel. This is natural remineralization.
The Fluoride Upgrade: Creating “Super-Teeth”
Here is where the importance of fluoride for dental health becomes chemically evident. When fluoride is present in the saliva during the remineralization process, it doesn’t just help replace the lost minerals. The fluoride ions actually substitute themselves for the hydroxyl ions in the crystal structure of the tooth.
This substitution changes the mineral from hydroxyapatite to fluorapatite. This is not a trivial change. Fluorapatite is a superior mineral structure for three main reasons:
- Increased Hardness: The crystal structure is tighter and more stable.
- Lower Critical pH: While standard enamel dissolves at a pH of 5.5, fluorapatite can withstand acidity down to a pH of 4.5. This means your teeth can survive stronger acid attacks without decaying.
- Bacterial Inhibition: Fluoride ions released from plaque interfere with the enzymes bacteria use to metabolize sugar. This effectively starves the bacteria and reduces acid production.
In my practice, I explain to patients that using fluoride is like reinforcing a brick wall with steel beams. You aren’t just repairing the wall. You are upgrading it to withstand forces that would have previously destroyed it. This enamel strengthening capability is unique to fluoride.
Expert Insight: The “White Spot” Warning
Have you ever noticed chalky white spots on teeth? They often appear near the gum line. These are often the first visible signs of demineralization. The enamel is still intact. However, it has lost minerals underneath the surface. This is the critical window where remineralization therapy using high-concentration fluoride can reverse the damage before a drill is ever needed.
Systemic vs. Topical Fluoride: A Dual-Action Defense
One of the most common points of confusion regarding the importance of fluoride for dental health is the method of delivery. We categorize fluoride into two distinct types based on how the body interacts with it. These are systemic and topical. Understanding the difference is essential for maximizing dental caries prevention.
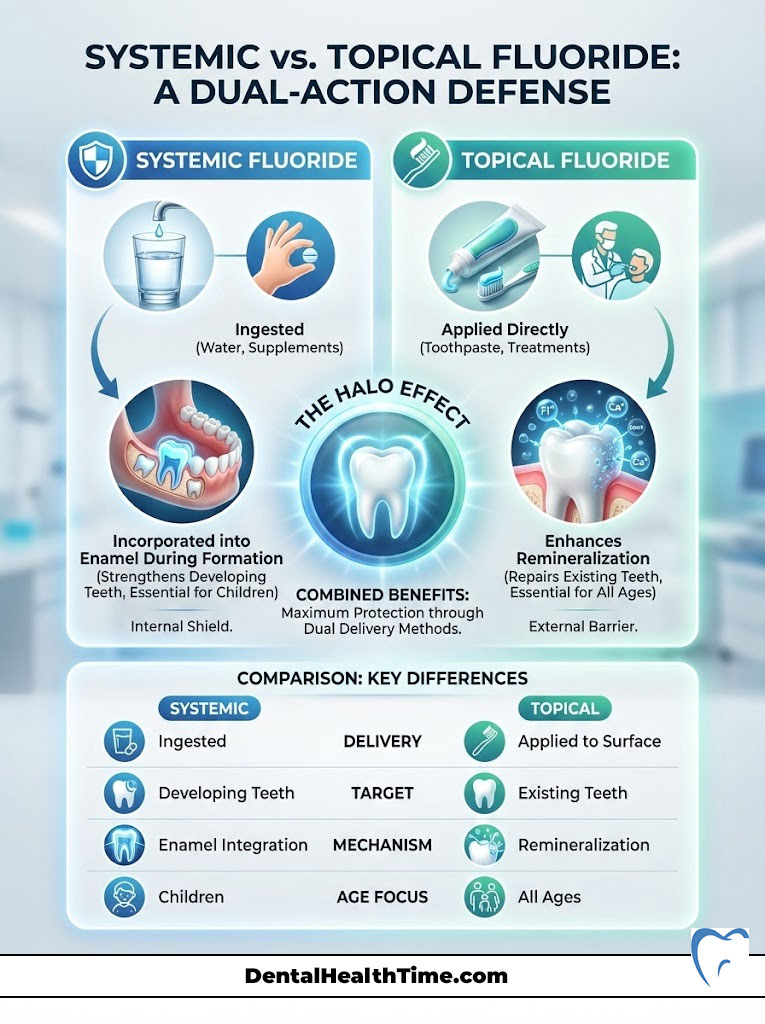
Systemic Fluoride: Building Strength from Within
Systemic fluoride is ingested. The primary sources are fluoridated community water and dietary supplements prescribed by pediatricians or dentists. When fluoride is ingested, it enters the bloodstream. It is then deposited into developing teeth that have not yet erupted through the gums.
For children, this is vital. As the permanent teeth form in the jawbone, systemic fluoride is incorporated directly into the enamel matrix. This means the tooth emerges already fortified with fluorapatite. It provides a baseline of protection that lasts a lifetime. However, once the teeth have erupted, the systemic benefit decreases significantly. Yet, secreted saliva containing ingested fluoride still provides some topical benefit.
Topical Fluoride: The Daily Shield
Topical fluoride is applied directly to the surface of the teeth. This comes from toothpaste, mouth rinses, and professional treatments like varnish or gel. The goal of topical fluoride is to maintain a low but constant concentration of fluoride in the saliva and plaque fluid.
When you brush with fluoride toothpaste, the concentration of fluoride in your mouth spikes. The enamel absorbs this fluoride to facilitate remineralization. Even after you rinse, a small amount remains in the saliva and soft tissues. This creates a reservoir that releases fluoride slowly over several hours. For adults, whose teeth are already fully formed, topical vs systemic fluoride debates are settled. Topical application is the primary driver of dental health.
The Halo Effect
The greatest protection comes from the “Halo Effect.” This is the combination of both delivery methods. Drinking fluoridated water provides a baseline. Daily brushing with fluoride toothpaste provides the high-concentration spikes needed to combat daily sugar intake. This dual approach is why we have seen such a dramatic decline in tooth decay over the last half-century.
Comparison: Systemic vs. Topical Fluoride Mechanisms
| Feature | Systemic Fluoride | Topical Fluoride |
|---|---|---|
| Primary Source | Community water, dietary supplements | Toothpaste, varnish, gels, rinses |
| Target Audience | Primarily children with developing teeth (0–16 years) | All ages (Children, Adults, Seniors) |
| Mechanism | Incorporated into the enamel matrix during formation | Adsorbs to surface enamel; inhibits bacterial enzymes |
| Duration of Effect | Permanent structural integration | Temporary surface protection (requires daily renewal) |
| Benefit for Adults | Topical effect via saliva secretion | Direct remineralization of exposed root surfaces |
Community Water Fluoridation (CWF): The Public Health Standard
When discussing the importance of fluoride for dental health, we must address one of the greatest public health achievements of the 20th century. This is community water fluoridation. This initiative began in Grand Rapids, Michigan, in 1945. The results changed the trajectory of dentistry forever.

The Data Behind the Strategy
Before fluoridation, tooth decay was almost universal. Nearly everyone had lost teeth by the time they reached middle age. Today, the landscape is vastly different. Data from the Centers for Disease Control and Prevention (CDC) consistently shows that fluoridated water reduces tooth decay by about 25% in children and adults. This reduction occurs regardless of socioeconomic status. This makes CWF a powerful tool for health equity.
The economic argument is equally compelling. The Return on Investment (ROI) for water fluoridation is staggering. For larger communities, every dollar spent on fluoridation saves roughly $20 in avoided dental treatment costs. In the U.S. alone, this amounts to billions of dollars saved annually by the healthcare system and families. It is a rare example of a public health intervention that actually saves money while improving quality of life.
Social Equity and Access
Not everyone has access to regular dental care or high-quality dental products. Low-income families are disproportionately affected by dental disease. This can lead to pain, missed school days, and systemic health issues. Community water fluoridation acts as a “great equalizer.”
By simply drinking tap water, individuals in underserved communities receive a baseline level of protection against decay. This passive intervention requires no behavior change. It requires no extra cost to the individual. It requires no daily effort. This ensures that the benefits of fluoride for teeth reach those who need them most.
Safety Standards and Toxicology: Addressing Modern Concerns
Despite the overwhelming clinical evidence supporting the importance of fluoride for dental health, I frequently encounter patients who are worried about toxicity. These concerns often stem from internet searches or misinterpreted studies. It is vital to address these fears with transparency and scientific context.
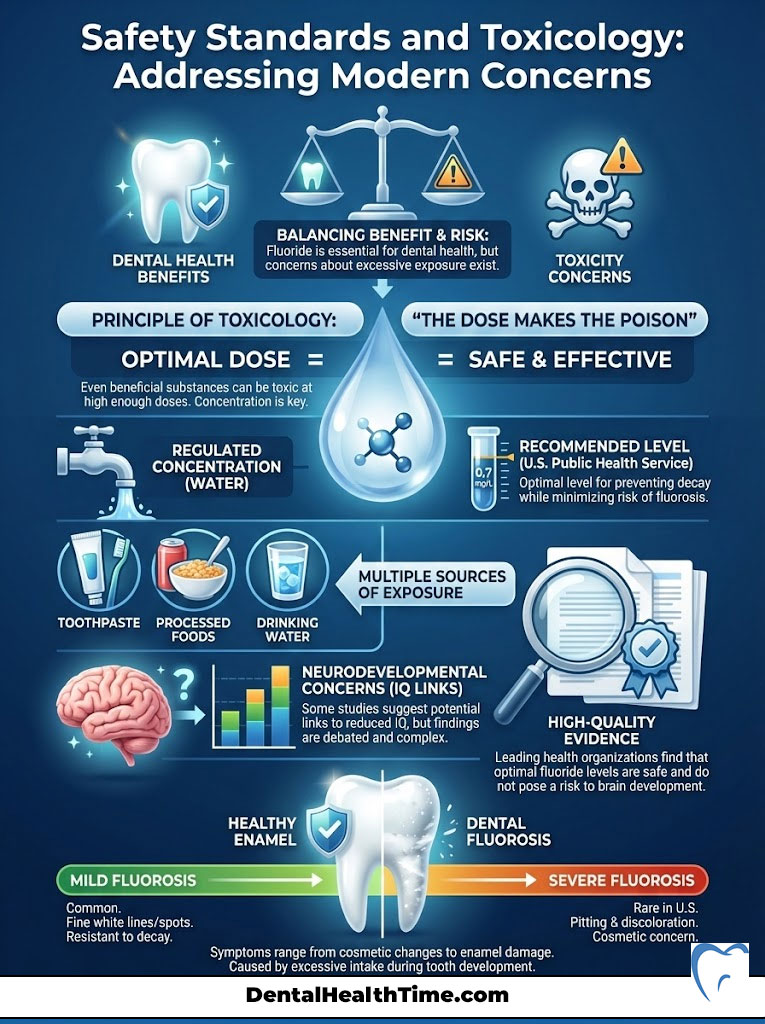
The Dose Makes the Poison
In toxicology, there is a fundamental principle: “The dose makes the poison.” This applies to everything we consume. This includes water, salt, and vitamins. Fluoride is no exception. At very high concentrations, fluoride can be toxic. However, the levels used in dentistry and water fluoridation are carefully regulated to be therapeutic, not toxic.
The U.S. Public Health Service recommends an optimal fluoride concentration in water of 0.7 milligrams per liter (mg/L). This level was adjusted downward from a range of 0.7–1.2 mg/L in 2015. This adjustment accounted for the fact that Americans now get fluoride from multiple sources. These include toothpaste and processed foods.
Addressing Neurodevelopmental Concerns
Recent discussions have centered on reports from the National Toxicology Program (NTP) regarding fluoride and IQ. It is essential to look at the details of these reviews. The studies that suggest a link between fluoride and lower IQ typically involve populations exposed to fluoride levels significantly higher than the 0.7 mg/L standard found in U.S. community water. Often these levels are two to three times higher. Current high-quality evidence supports the safety of fluoride at the optimal levels used in the United States.
Dental Fluorosis: A Cosmetic Nuance
The most common side effect of fluoride ingestion is dental fluorosis. This occurs when a child ingests too much fluoride while their teeth are forming under the gums. This usually happens under age 8. The symptoms of dental fluorosis are typically mild. They appear as faint white lines or streaks on the enamel surface.
In the vast majority of cases in the U.S., fluorosis is barely visible to the naked eye. It does not affect the function of the tooth. In fact, teeth with mild fluorosis are often more resistant to decay. Severe fluorosis, which can cause pitting or discoloration, is extremely rare in communities with controlled water fluoridation. It is usually associated with naturally high fluoride levels in untreated well water or accidental ingestion of large amounts of toothpaste over time.
Fluoride Guidelines by Life Stage
The importance of fluoride for dental health evolves as we age. The strategies we use for a toddler are different from those we use for a senior citizen. Here is a breakdown of how to manage fluoride exposure safely and effectively at every stage of life.
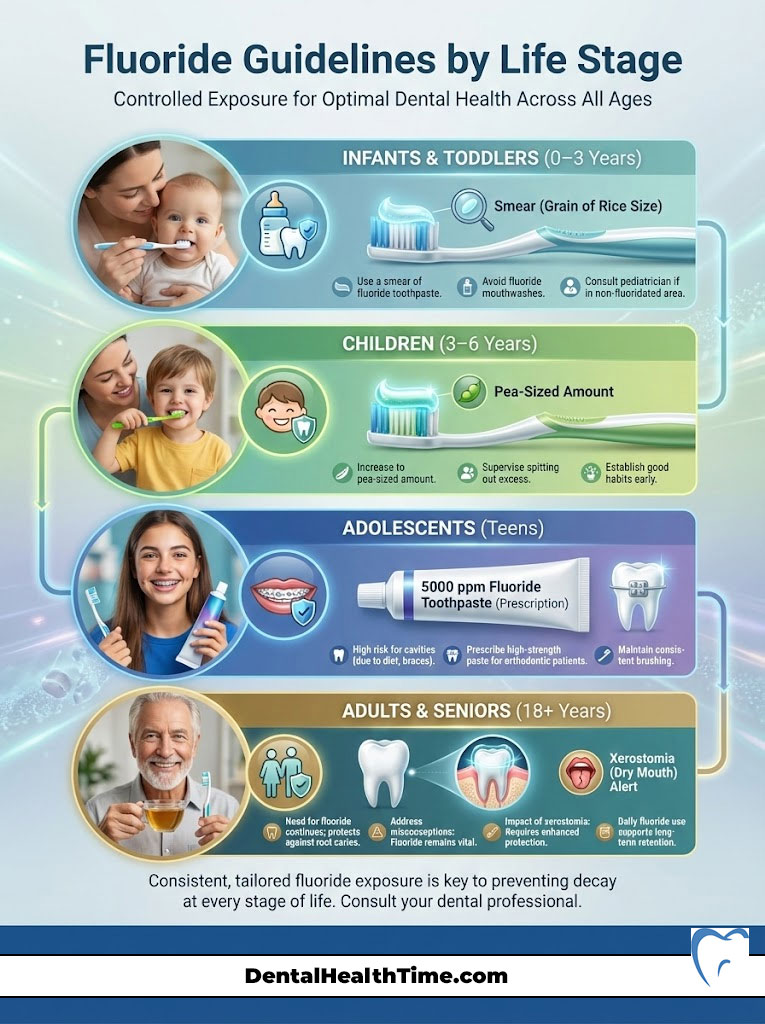
Infants and Toddlers (0–3 Years)
The primary goal here is prevention without over-ingestion. As soon as the first tooth erupts, the American Academy of Pediatrics and the ADA recommend using a “smear” of fluoride toothpaste. This should be about the size of a grain of rice. This tiny amount provides the necessary topical benefit while minimizing the risk if the child swallows it.
Parents often ask, “Is fluoride safe for toddlers?” Yes, absolutely, provided the dose is controlled. Avoid fluoride mouthwashes at this age. Children cannot yet spit effectively.
Children (3–6 Years)
At this stage, children can usually spit. However, they still need supervision. Increase the amount of toothpaste to the size of a pea. Parents should dispense the toothpaste. Encourage the child to spit out the excess foam.
This is the critical window for preventing fluorosis on the developing permanent front teeth. Therefore, ensuring they don’t swallow the paste is key. If you live in a non-fluoridated area, consult your pediatrician about fluoride supplements.
Adolescents: The Orthodontic Years
Teens are at high risk for cavities due to dietary habits and orthodontic treatment. Braces create hiding spots for bacteria. This leads to rapid demineralization. We often see “white spot lesions” form around brackets in a matter of months.
For teens with braces, I often prescribe a high-concentration fluoride toothpaste (5000 ppm) to use at night. This prescription-strength paste provides extra enamel strengthening power. It counteracts the difficulty of cleaning around wires.
Adults and Seniors
Many adults believe they “age out” of needing fluoride. This is a dangerous misconception. As we age, gums recede. This exposes the tooth roots. Unlike the crown, which is covered in hard enamel, roots are covered in cementum and dentin. These tissues are much softer and decay much faster.
Fluoride treatment for adults is critical to preventing root caries. Furthermore, many seniors suffer from Xerostomia (dry mouth) caused by medications. Without saliva to naturally buffer acid, teeth can be destroyed rapidly. For these patients, high-fluoride therapies are not just optional. They are the only line of defense remaining.
Professional Fluoride Treatments: Varnish, Gel, and SDF
While daily home care is the foundation, professional treatments provided in the dental office offer a concentrated boost that home products cannot match. The importance of fluoride for dental health is amplified when we use professional-grade materials.
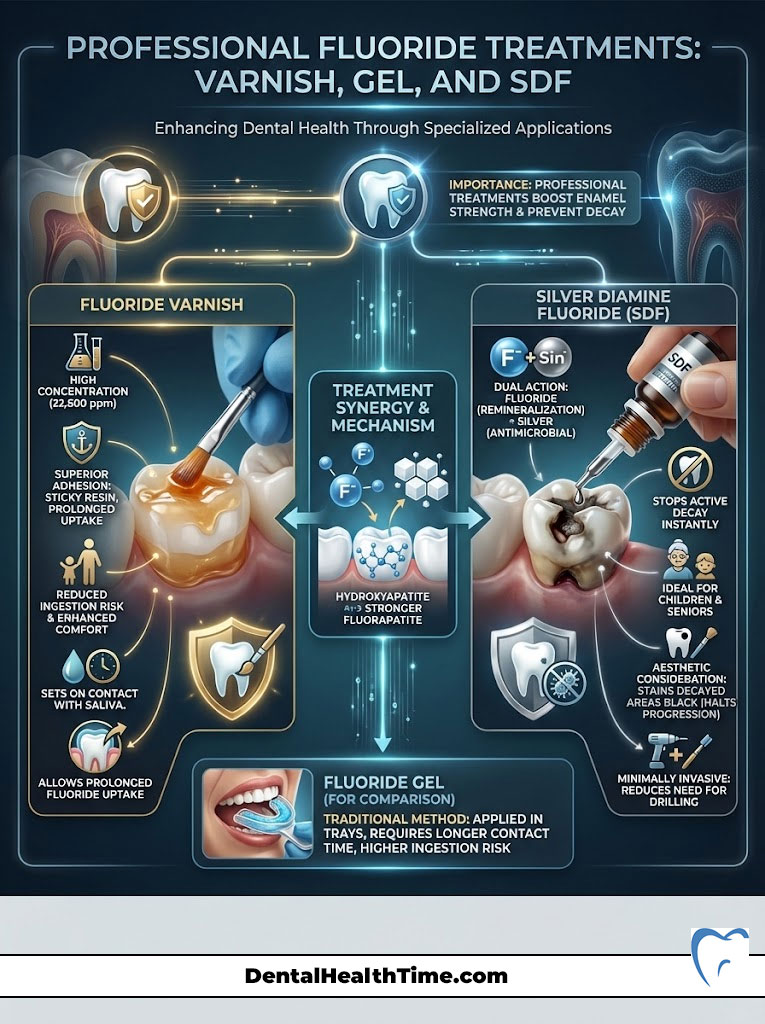
Fluoride Varnish
Gone are the days of biting into messy foam trays for minutes on end. Modern dentistry primarily uses fluoride varnish. This is a sticky, resin-based material painted directly onto the teeth. It contains a high concentration of fluoride (typically 22,600 ppm).
The varnish sets on contact with saliva. It remains on the teeth for several hours. This allows for a deep, prolonged uptake of fluoride into the enamel. Fluoride varnish benefits include superior adhesion, reduced risk of ingestion, and better patient comfort.
Silver Diamine Fluoride (SDF)
SDF is a game-changer in minimally invasive dentistry. It combines the remineralizing power of fluoride with the antimicrobial properties of silver. We use SDF to halt active decay instantly. It is particularly useful for young children who cannot sit for a filling.
It is also excellent for seniors with extensive decay who have medical limitations. While it can stain the decayed area black, it effectively stops the cavity from growing. This often eliminates the need for drilling.
Fluoride vs. Nano-Hydroxyapatite: The Modern Comparison
In recent years, a new contender has entered the oral health market: Nano-Hydroxyapatite (n-Ha). Many patients ask if they can switch to n-Ha to avoid fluoride while still protecting their teeth. To understand the importance of fluoride for dental health in this context, we must compare the two materials objectively.
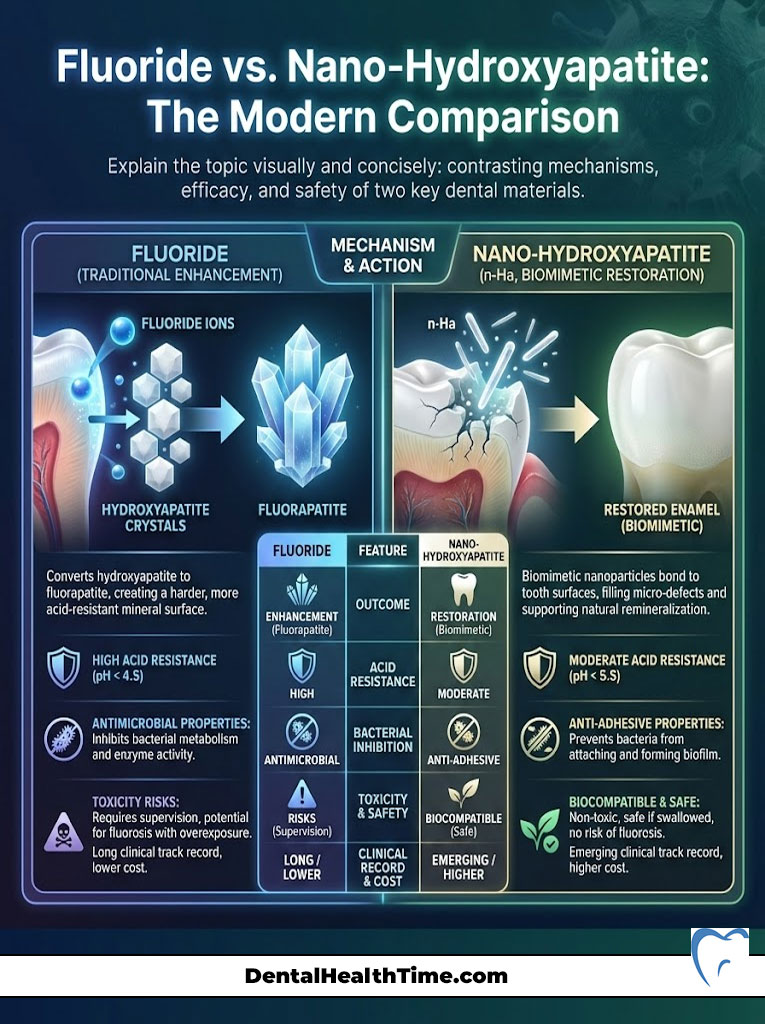
What is Nano-Hydroxyapatite?
Nano-Hydroxyapatite is a biomimetic material. It mimics the natural structure of tooth enamel. When you brush with n-Ha, the particles bond to the tooth surface. They fill in microscopic tubules and replace lost minerals. It is effective at reducing sensitivity and supporting remineralization.
The Verdict: Enhancement vs. Restoration
While n-Ha is a valid technology, there is a distinct difference in outcome. Nano-Hydroxyapatite restores the tooth to its original strength (hydroxyapatite). Fluoride, however, converts the tooth surface into fluorapatite.
Fluorapatite is stronger and more acid-resistant than the original tooth. Therefore, fluoride provides an enhancement beyond baseline biology. Conversely, n-Ha provides restoration to baseline. For patients who strictly refuse fluoride, n-Ha is the best alternative. However, for maximum dental caries prevention, particularly in patients with high sugar intake or dry mouth, fluoride remains the gold standard. The hydroxyapatite vs fluorapatite debate ultimately favors fluoride for acid resistance.
Comparison: Fluoride vs. Nano-Hydroxyapatite (n-Ha)
| Attribute | Fluoride (Sodium / Stannous) | Nano-Hydroxyapatite (n-Ha) |
|---|---|---|
| Chemical Action | Converts hydroxyapatite to acid-resistant fluorapatite | Remineralizes by bonding synthetic hydroxyapatite to enamel |
| Acid Resistance | High (Critical pH ~4.5) | Moderate (Critical pH ~5.5) |
| Bacterial Inhibition | Disrupts bacterial enzyme activity (anti-microbial) | Prevents bacterial adhesion (anti-adhesive) |
| Toxicity Risk | Toxic if ingested in large quantities (requires supervision) | Biocompatible and non-toxic if swallowed |
| Clinical Track Record | 75+ years of extensive clinical data | ~40 years of data (popularized recently) |
| Cost | Low (widely available) | Higher (specialty product) |
Types of Fluoride in Toothpaste: Sodium vs. Stannous
Walking down the toothpaste aisle can be overwhelming. You will see various active ingredients listed on the boxes. Two of the most common are Sodium Fluoride and Stannous Fluoride. Understanding the difference can help you choose the right product for your specific needs.
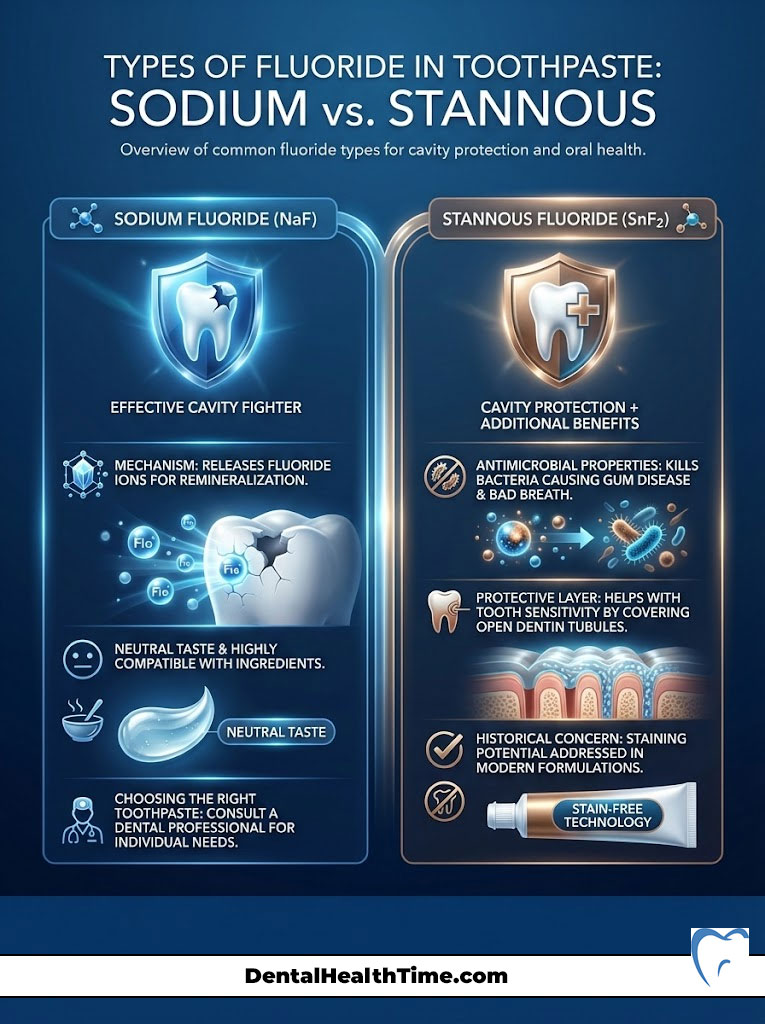
Sodium Fluoride (NaF)
This is the classic cavity fighter. Sodium fluoride breaks down easily in the mouth to release fluoride ions. These ions are then available for the remineralization process we discussed earlier. It is highly effective at preventing decay. It has a neutral taste. It is generally compatible with most other toothpaste ingredients.
Stannous Fluoride (SnF2)
Stannous fluoride is a powerhouse ingredient. It provides the same cavity protection as sodium fluoride. However, it offers additional benefits. The “stannous” component (tin) has antimicrobial properties.
This means it kills bacteria that cause gum disease (gingivitis) and bad breath. It also creates a protective layer over open dentin tubules. This makes it excellent for treating tooth sensitivity. Historically, stannous fluoride could stain teeth. However, modern formulations have largely stabilized the ingredient to prevent this issue.
Global Perspectives on Fluoridation
Critics often point out that many European countries do not fluoridate their water. They use this as an argument against the practice. However, this is a misunderstanding of public health logistics. The goal of dental caries prevention is universal. The method of delivery simply changes based on infrastructure.
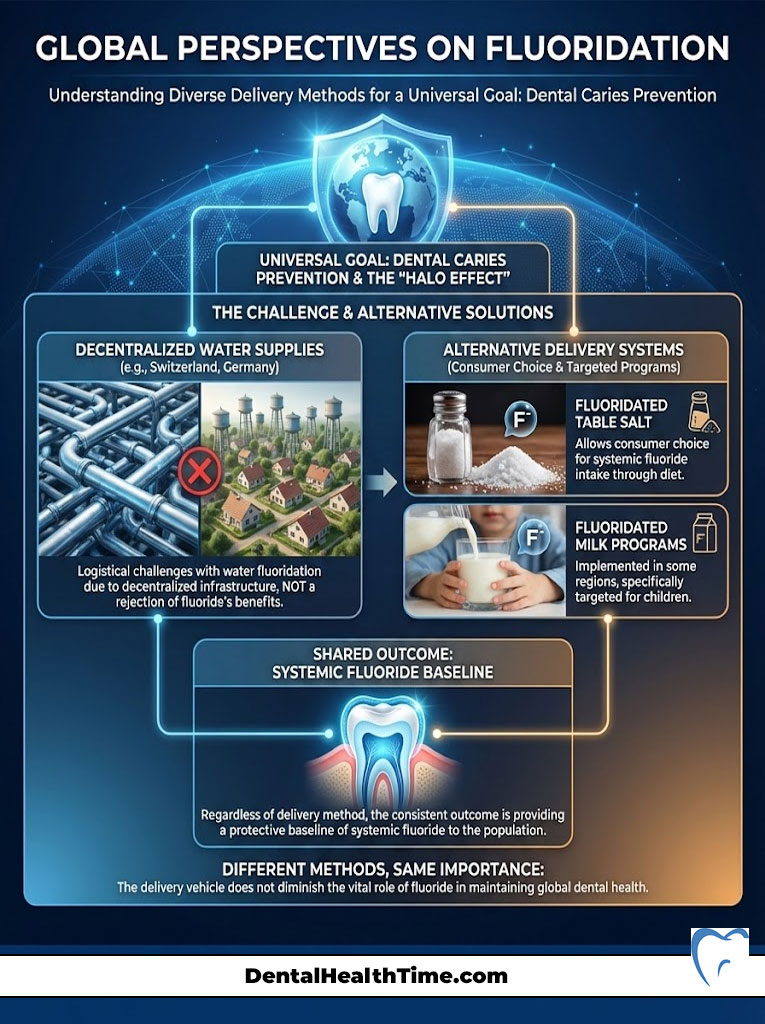
Salt and Milk Fluoridation
In countries like Switzerland and Germany, water fluoridation is technically difficult due to decentralized water supplies. Instead, these nations use fluoridated table salt. This allows consumers to choose whether they want systemic fluoride. Other regions have experimented with fluoridated milk programs for children.
The outcome is the same: providing a baseline of systemic fluoride to the population. The fact that different countries use different vehicles does not negate the importance of fluoride for dental health. It simply highlights that there are multiple ways to achieve the same protective “Halo Effect.”
Advanced Home Care Routines for High-Risk Patients
For some patients, standard brushing and flossing are not enough. If you have a history of frequent cavities, dry mouth, or heavily restored teeth, you fall into a “high-risk” category. Here is an advanced protocol I recommend to maximize the benefits of fluoride for teeth.
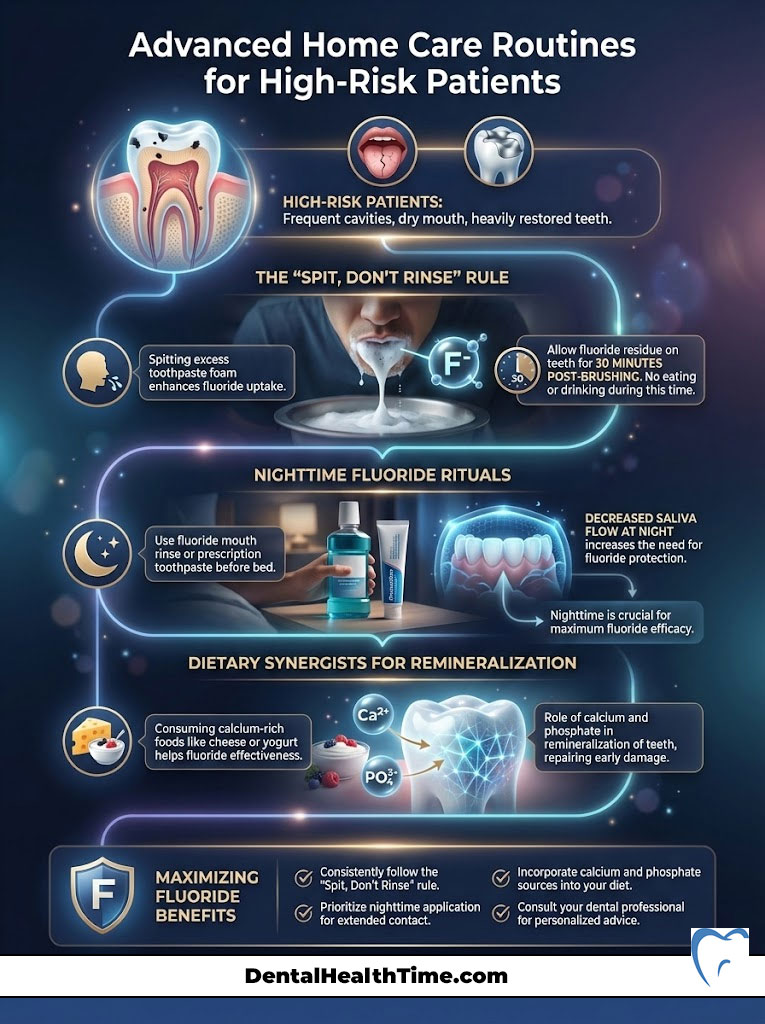
The “Spit, Don’t Rinse” Rule
Most people brush their teeth and then immediately rinse with water. This washes away the beneficial fluoride before it has time to work. Instead, spit out the excess toothpaste foam but do not rinse with water. Let the residue sit on your teeth for at least 30 minutes. This significantly increases fluoride uptake.
Nighttime Fluoride Rituals
Saliva flow decreases while you sleep. This reduces your natural defense against acid. Therefore, nighttime is the most critical time for fluoride application. Using a fluoride mouth rinse before brushing can help. Or, using a prescription-strength fluoride toothpaste as your final step before bed can provide 8 hours of uninterrupted protection.
Dietary Synergists
Fluoride works best when there is ample calcium and phosphate available in the saliva. Consuming cheese or yogurt after a sugary snack can help neutralize acid and provide the raw materials needed for remineralization. This creates a perfect environment for fluoride to do its job.
Summary & Key Takeaways
The importance of fluoride for dental health is not merely a matter of opinion. It is a scientifically proven reality that has improved the quality of life for millions. From the molecular transformation of hydroxyapatite into robust fluorapatite to the large-scale economic benefits of community water fluoridation, fluoride stands as the cornerstone of modern preventive dentistry.
We have explored how fluoride works to combat demineralization. We have analyzed the synergy between topical and systemic delivery. We have also seen why it remains superior to modern alternatives for acid resistance. While safety concerns are valid, the data confirms that when used at optimal levels, fluoride is safe, effective, and essential.
To maximize the benefits of fluoride for teeth, remember these core principles:
- Utilize the “Halo Effect” by drinking fluoridated water and using fluoride toothpaste.
- Supervise young children to prevent fluorosis while ensuring they get the protective benefits.
- Don’t stop fluoride use as you age; adults need it to prevent root caries and manage dry mouth.
- Consult your dentist about professional treatments like varnish if you are at high risk for decay.
- Understand that alternative products like n-Ha are good, but fluoride offers superior acid resistance.
By integrating these practices into your daily life, you ensure that your smile remains not just visually appealing, but structurally sound for decades to come.
Frequently Asked Questions
How does fluoride facilitate the remineralization of tooth enamel?
Fluoride works by pairing with calcium and phosphate ions in your saliva to rebuild weakened enamel. During this process, it replaces the hydroxyl group in the tooth’s mineral structure, creating fluorapatite. This new crystalline structure is significantly harder and more resistant to acid attacks than the original hydroxyapatite.
What is the difference between systemic and topical fluoride applications?
Systemic fluoride is ingested through water or supplements and is incorporated into the enamel matrix of developing teeth before they erupt. Topical fluoride, found in toothpaste and professional varnishes, provides a direct surface defense for erupted teeth. Both methods work synergistically to create a “halo effect” that provides comprehensive protection across all life stages.
Is community water fluoridation safe for long-term health?
Yes, community water fluoridation is supported by over 70 years of clinical research and is recognized by the CDC as a top public health achievement. The current recommended concentration of 0.7 mg/L is carefully regulated to provide maximum dental benefits while remaining well below levels associated with adverse health effects. It serves as a cost-effective and equitable way to reduce tooth decay by approximately 25% across entire populations.
Do adults and seniors still benefit from fluoride treatments?
Adults benefit immensely from fluoride, particularly as they age and experience gingival recession. Exposed tooth roots are covered in cementum, which is softer than enamel and highly susceptible to rapid decay. Fluoride treatments for adults help prevent these root caries and provide a necessary defense for those suffering from xerostomia, or dry mouth, caused by various medications.
What causes dental fluorosis and how can it be prevented in children?
Dental fluorosis is a cosmetic condition caused by excessive fluoride ingestion while permanent teeth are still forming under the gums, typically before age eight. To prevent this, parents should use only a “smear” of toothpaste for children under three and a pea-sized amount for those aged three to six. Supervision is key to ensuring children spit out the excess toothpaste rather than swallowing it.
How does fluoride compare to nano-hydroxyapatite for enamel protection?
While nano-hydroxyapatite is an effective biomimetic material that restores enamel to its baseline strength, fluoride actually upgrades the tooth’s biology. Fluoride converts enamel into fluorapatite, which has a lower critical pH of 4.5 compared to the standard 5.5. This means fluoridated teeth can withstand significantly more acidic environments before demineralization begins.
What are the advantages of stannous fluoride over sodium fluoride?
Both are excellent cavity fighters, but stannous fluoride offers additional antimicrobial benefits that target gingivitis and oral bacteria. The tin ion in stannous fluoride helps inhibit bacterial enzymes and creates a protective layer over dentin tubules to reduce tooth sensitivity. Sodium fluoride is a more traditional, neutral-tasting option focused primarily on the remineralization of enamel.
Why is the spit, don’t rinse rule recommended after brushing?
Rinsing with water immediately after brushing washes away the concentrated fluoride ions before they can be absorbed by the enamel. By simply spitting out the excess foam and avoiding liquids for 30 minutes, you allow the fluoride to maintain a therapeutic concentration in your saliva. This prolonged contact time is essential for the chemical conversion of hydroxyapatite into acid-resistant fluorapatite.
What is Silver Diamine Fluoride (SDF) and when is it used?
SDF is a liquid medication used to instantly halt the progression of active dental caries through a combination of antimicrobial silver and high-concentration fluoride. It is an invaluable tool for pediatric patients, seniors, or individuals with dental anxiety who may not be able to tolerate traditional drilling and filling procedures. While it can cause dark staining on the decayed area, it effectively “freezes” the cavity and prevents further destruction.
Can fluoride help reverse early signs of tooth decay?
Yes, fluoride is highly effective at reversing incipient lesions, which often appear as chalky white spots on the teeth. These spots indicate that the enamel has lost minerals but the surface structure is still intact. High-concentration topical fluoride treatments can drive minerals back into these porous areas, hardening the enamel and preventing the need for a traditional filling.
How do professional fluoride varnishes differ from over-the-counter products?
Professional varnishes contain a much higher concentration of fluoride, typically around 22,600 ppm, compared to the 1,000–1,500 ppm found in standard toothpaste. The resin-based varnish is designed to adhere to the tooth surface for several hours, providing a deep, sustained release of fluoride. This intensive treatment is especially beneficial for high-risk patients, such as those with orthodontic braces or chronic dry mouth.
Is fluoride exposure linked to lower IQ in children?
Current high-quality scientific evidence supports the safety of fluoride at the optimal levels used in U.S. community water systems (0.7 mg/L). Most studies suggesting a link between fluoride and neurodevelopmental issues involve populations exposed to levels two to three times higher than the U.S. standard. When used as directed, fluoride remains one of the safest and most effective tools in preventive dentistry.
Disclaimer
This article is for informational purposes only and does not constitute medical or dental advice. While fluoride is a widely researched and recommended mineral for oral health, individual needs may vary based on medical history and geographic location. Always consult with a qualified dentist or healthcare professional before changing your dental routine or starting new supplements.
References
- Centers for Disease Control and Prevention (CDC) – Community Water Fluoridation Resources – Provides comprehensive data on the 25% reduction in tooth decay and public health statistics.
- American Dental Association (ADA) – Fluoride in Water – Clinical guidelines on the safety and efficacy of fluoride for all age groups.
- World Health Organization (WHO) – Oral Health Fact Sheets – Global perspectives on dental caries prevention and the role of fluoride in salt and water.
- National Institute of Dental and Craniofacial Research (NIDCR) – The Story of Fluoridation – Detailed scientific breakdown of the remineralization process and fluorapatite formation.
- American Academy of Pediatrics (AAP) – HealthyChildren.org – Guidelines for fluoride use in infants and toddlers to prevent early childhood caries.
- Journal of Dental Research – Clinical Studies on Stannous vs. Sodium Fluoride – Peer-reviewed research comparing the antimicrobial benefits of different fluoride compounds.