Learning how to choose the right toothpaste requires ignoring the marketing on the front of the box and analyzing the ingredient list on the back. For effective cavity prevention, your product must contain a remineralizing agent like fluoride (1,000–1,500 ppm) or nano-hydroxyapatite. If you suffer from sharp pain when drinking cold water, you need potassium nitrate to calm the nerve or stannous fluoride to block the tubules.
Table of Contents
Always check the Relative Dentin Abrasivity (RDA); keep it under 70 to prevent enamel erosion. Finally, ensure the product carries the ADA Seal of Acceptance to guarantee it is free from harmful contaminants and delivers the therapeutic dosage promised.

Key Dental Statistics
- 90% of the adult population has experienced at least one carious lesion (cavity).
- 1 in 8 individuals suffers from chronic dentin hypersensitivity due to enamel loss.
- RDA 250 is the absolute safety limit set by the FDA; however, dentists recommend staying below 150.
- 2 minutes is the minimum contact time required for fluoride to chemically bind to enamel.
- 40% of toothpaste volume is simply water and humectants, not active cleaning agents.
- 5.5 pH is the critical threshold where enamel begins to dissolve; your toothpaste must buffer above this level.
The Science Behind the Smile
You are standing in aisle four of the local pharmacy. The fluorescent lights hum above you. Before you stands a daunting wall of colorful boxes. They promise everything from blinding white smiles to the total regeneration of your enamel. It is paralyzing. As a clinician, I witness this confusion nearly every day. Patients frequently select a product based on the graphic design or a promise of “minty freshness.” However, knowing how to choose the right toothpaste is a medical decision. It should not be a cosmetic one.

Toothpaste, technically known as dentifrice, is a sophisticated drug delivery system. Its purpose is threefold. First, it must reduce the bacterial load in your oral microbiome. Second, it must fortify the mineral structure of your teeth against acid attacks. Third, it must maintain the health of your gingival tissue. When you choose the wrong formulation, the consequences are tangible. I recently treated a patient who had scrubbed her enamel away using a trendy charcoal paste. She believed she was being “natural” and safe. In reality, she was using a product with the abrasive quality of fine sandpaper.
Marketing campaigns frequently contradict medical science. In this comprehensive guide, we will strip away the branding and examine the pharmacology. We will analyze the critical Relative Dentin Abrasivity (RDA) scale. We will compare the efficacy of nano-hydroxyapatite versus fluoride. We will determine exactly which ingredients belong in your dental care regimen. This is not just about cleaning your teeth; it is about preserving your biology.
The Pharmacology of Dentifrice: Deconstructing the Tube
To understand what you are buying, you must deconstruct the contents of the tube. Generally, about 50% of the paste consists of inactive carriers. These make the product squeezable, shelf-stable, and pleasant to taste. The other 50% comprises the active therapy that actually interacts with your hard and soft tissues. Let’s break down the anatomy of a typical tube.

Abrasives: The Mechanics of Cleaning
Abrasives are the workhorses of stain removal. Without them, your toothbrush would simply glide over the sticky biofilm without dislodging it. Common examples include hydrated silica, calcium carbonate, and dicalcium phosphate. They physically scrub the bacterial film and extrinsic stains off the tooth surface. The balance here is incredibly delicate. You need enough grit to clean the surface. However, too much grit will erode the enamel rods over time. This is where the Relative Dentin Abrasivity (RDA) score becomes the most vital metric you have never heard of.
Humectants and Binders
Have you ever wondered why toothpaste doesn’t dry out or separate into a watery mess? Humectants like sorbitol, glycerol, and propylene glycol retain moisture. They prevent the paste from hardening in the tube. Binders, such as xanthan gum, carrageenan, or cellulose gum, maintain the creamy texture. While these do not clean your teeth, they ensure the active ingredients remain suspended. This ensures that the first squeeze delivers the same dosage as the last squeeze.
Detergents: The Foaming Action
Many patients equate foam with cleanliness. This foaming action is usually caused by surfactants. The most common is Sodium Lauryl Sulfate (SLS). Another popular option is cocamidopropyl betaine. These surfactants lower the surface tension of saliva. This allows the paste to spread into interproximal spaces and loosen debris. However, SLS is a double-edged sword. It is a known irritant for many patients with soft tissue sensitivities. We will discuss the implications of SLS on oral ulcers later in this guide.
Flavoring and Sweeteners
Raw fluoride and detergents taste bitter and soapy. Flavoring agents are essential for patient compliance. If it tastes bad, you won’t brush for the full two minutes. Common sweeteners include saccharin and sorbitol. However, Xylitol is the gold standard. Xylitol is a natural sugar alcohol that bacteria cannot digest. It actually starves the harmful bacteria, adding a therapeutic benefit to the flavor profile.
Dr. Sterling’s Insight: The ADA Council on Scientific Affairs requires that any toothpaste bearing the ADA Seal of Acceptance must contain fluoride. If you see the seal, you know the product has met rigorous safety and efficacy standards for cavity prevention. It also guarantees that the manufacturing facility meets FDA pharmaceutical standards.
The Remineralization Debate: Fluoride vs. Nano-Hydroxyapatite
The primary function of any therapeutic toothpaste is to combat acid erosion. Bacteria in your mouth consume dietary sugars and produce lactic acid. This acid pulls minerals (calcium and phosphate) out of your teeth. Your toothpaste must put them back in. This process is called remineralization. Without it, the tooth structure collapses, and a cavity forms.
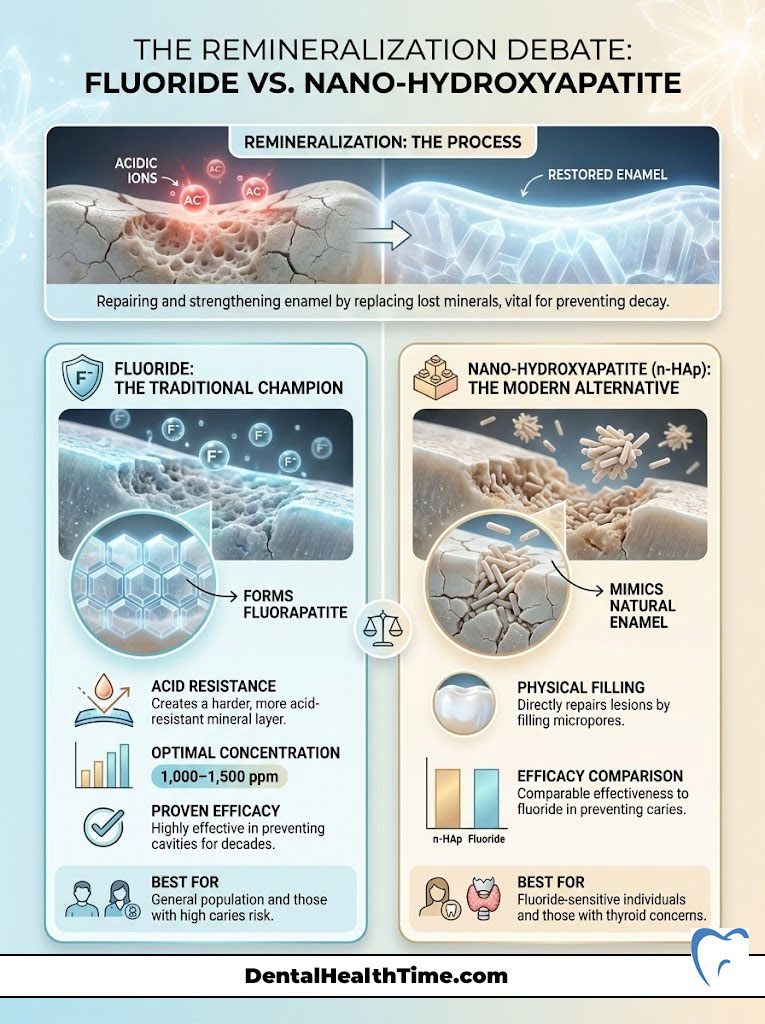
The Gold Standard: Fluoride
For decades, fluoride has been the undisputed king of cavity prevention. Its mechanism is chemically elegant. It interacts with the hydroxyapatite crystals in your enamel. It replaces the hydroxyl group to form fluorapatite. This new compound is significantly more resistant to acid attacks than your natural enamel. It lowers the critical pH at which teeth dissolve.
There are three main types you will encounter on labels:
- Sodium Fluoride (NaF): This is the most common form found in generic pastes. It releases fluoride ions instantly upon contact with saliva. It is highly effective but requires compatible abrasives (usually silica) to remain stable in the tube. It is excellent for strengthening enamel.
- Sodium Monofluorophosphate (SMFP): This compound holds onto the fluoride ion more tightly. It requires enzymatic breakdown in the mouth to release the fluoride. It is often used with calcium-based abrasives because it does not react prematurely with the calcium.
- Stannous Fluoride (SnF2): This is the multi-tasker. It provides fluoride for cavities, but the “stannous” (tin) component is antimicrobial. It kills bacteria and blocks pain channels. Historically, it stained teeth, but modern stabilization has resolved this issue.
For adult efficacy, I recommend a concentration between 1,000 to 1,500 ppm (parts per million). Anything less is unlikely to provide significant protection against a modern high-sugar diet.
The Modern Contender: Nano-Hydroxyapatite (n-HAp)
In recent years, a new player has emerged from Japan and is gaining massive traction in the US. It is called nano-hydroxyapatite (n-HAp). This ingredient is biomimetic. This means it mimics your body’s biology. Your enamel is made of hydroxyapatite. This ingredient provides the exact raw material needed to repair the tooth.
Unlike fluoride, which causes a chemical reaction, n-HAp works by physically filling the microscopic lesions. It plugs the tubules in the tooth surface. It acts like spackle on a drywall crack. Clinical trials have shown that high-concentration n-HAp is comparable to fluoride in preventing caries. For patients who are strictly opposed to fluoride or have thyroid concerns, n-HAp is the only valid scientific alternative for remineralization of enamel. Do not confuse this with standard hydroxyapatite; the particle size must be “nano” to penetrate the enamel rods.
Understanding Abrasivity: The RDA Scale Explained
This is the most critical metric that is almost never printed on the box. The Relative Dentin Abrasivity (RDA) scale measures how erosive a toothpaste is to the dentin. Dentin is the yellow layer under your enamel. It is much softer than enamel. If you have gum recession, your dentin is exposed. Using a high-RDA toothpaste on exposed dentin is disastrous.
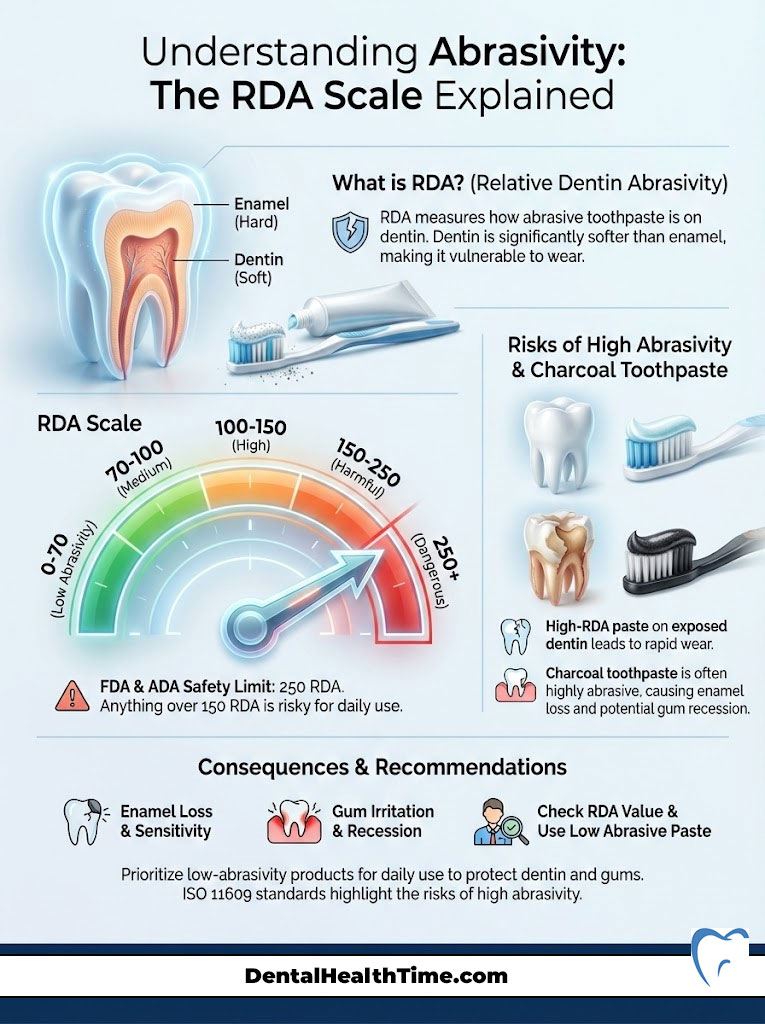
The Scale Breakdown
The FDA and ADA have set a safety limit of 250. However, in my professional opinion, anything over 150 is risky for daily use. Here is how the numbers translate to real-world safety:
- 0–70 (Low Abrasive): This is the safe zone. These pastes are ideal for patients with recession, exposed roots, or veneers. They are also necessary for those who use electric toothbrushes. Electric brushes add their own physical force, so you need a lower RDA paste to compensate.
- 70–100 (Medium Abrasive): This is the standard range for most major grocery store brands. It balances stain removal with safety for healthy adults. If you have thick enamel and no sensitivity, this range is acceptable.
- 100–150 (High Abrasive): Most “Whitening” and “Tartar Control” formulas fall here. They are effective at removing surface stains. However, they can cause sensitivity over time. Daily use can slowly thin the enamel, leading to translucency.
- 150–250 (Harmful Limit): Use these with extreme caution. I rarely recommend products in this range for daily long-term use. They act like a scrub. While your teeth may feel smooth, you are removing legitimate tooth structure.
The Charcoal Warning
Charcoal toothpaste is a marketing phenomenon, not a medical breakthrough. The premise is that charcoal absorbs toxins. In the mouth, however, it acts primarily as an abrasive. Many charcoal formulations test very high on the RDA scale. They scour the surface effectively. Unfortunately, they often take the enamel with them. Once enamel is gone, it does not grow back. The data from ISO 11609 standards testing confirms that high abrasivity leads to permanent surface loss. Furthermore, charcoal particles can lodge in the gum line, creating a “tattoo” effect or causing inflammation.
Comparison: Active Ingredients for Common Dental Goals
When determining how to choose the right toothpaste, you must align the active ingredient with your primary goal. Do not try to find one paste that does everything. Focus on your chief complaint. Use this table as your selection guide.
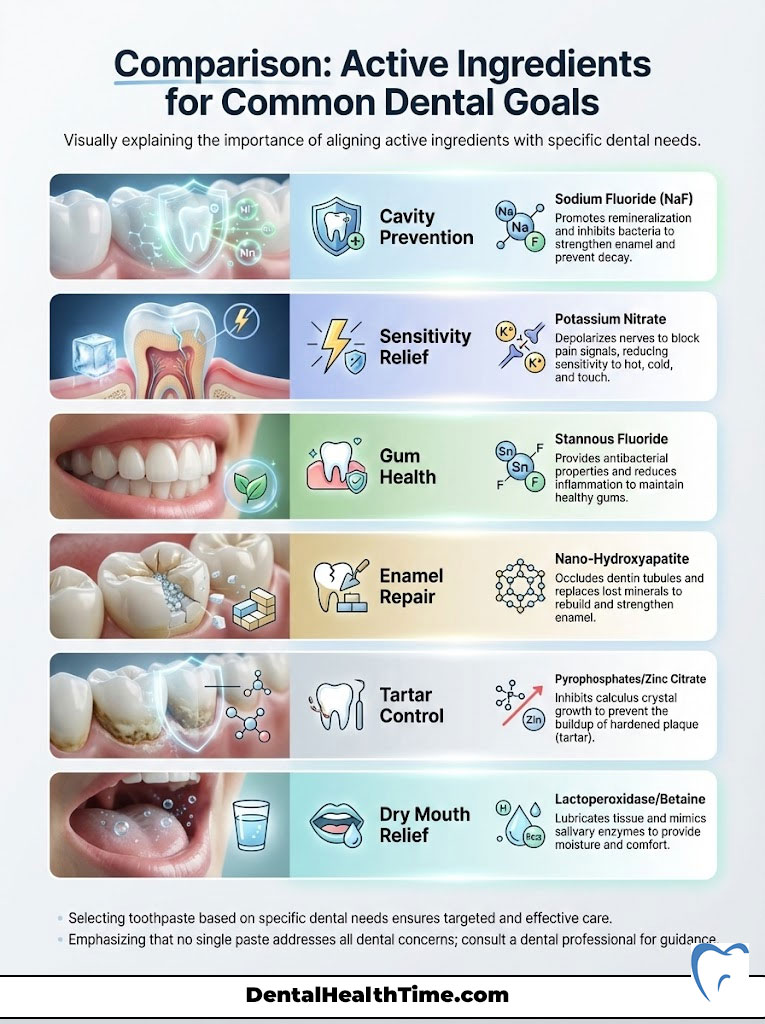
| Dental Goal | Primary Active Ingredient | Mechanism of Action | Recommended For |
|---|---|---|---|
| Cavity Prevention | Sodium Fluoride (NaF) | Promotes remineralization; inhibits bacterial enzymes | General population; high caries risk |
| Sensitivity Relief | Potassium Nitrate | Depolarizes the nerve; blocks pain signals to the brain | Patients with cold/hot sensitivity |
| Gum Health | Stannous Fluoride | Antibacterial; reduces biofilm and gingival inflammation | Gingivitis; bleeding gums; bad breath |
| Enamel Repair | Nano-Hydroxyapatite | Occludes dentin tubules; replaces lost minerals physically | Fluoride-free preference; early decay |
| Tartar Control | Pyrophosphates / Zinc Citrate | Inhibits crystal growth of calculus (tartar) | Heavy tartar builders; rapid stainers |
| Dry Mouth | Lactoperoxidase / Betaine | Lubricates tissue; mimics natural salivary enzymes | Sjogren’s syndrome; medication side effects |
Managing Dentin Hypersensitivity: The Science of Pain
If a sip of ice water sends a shockwave through your jaw, you are experiencing dentin hypersensitivity. This occurs when the microscopic tubules in your dentin are exposed. This exposure usually happens due to gum recession or enamel erosion from acid reflux or aggressive brushing. Fluid inside these tubules moves when temperature changes occur. This movement stimulates the nerve pulp. This is known as the Hydrodynamic Theory of pain.
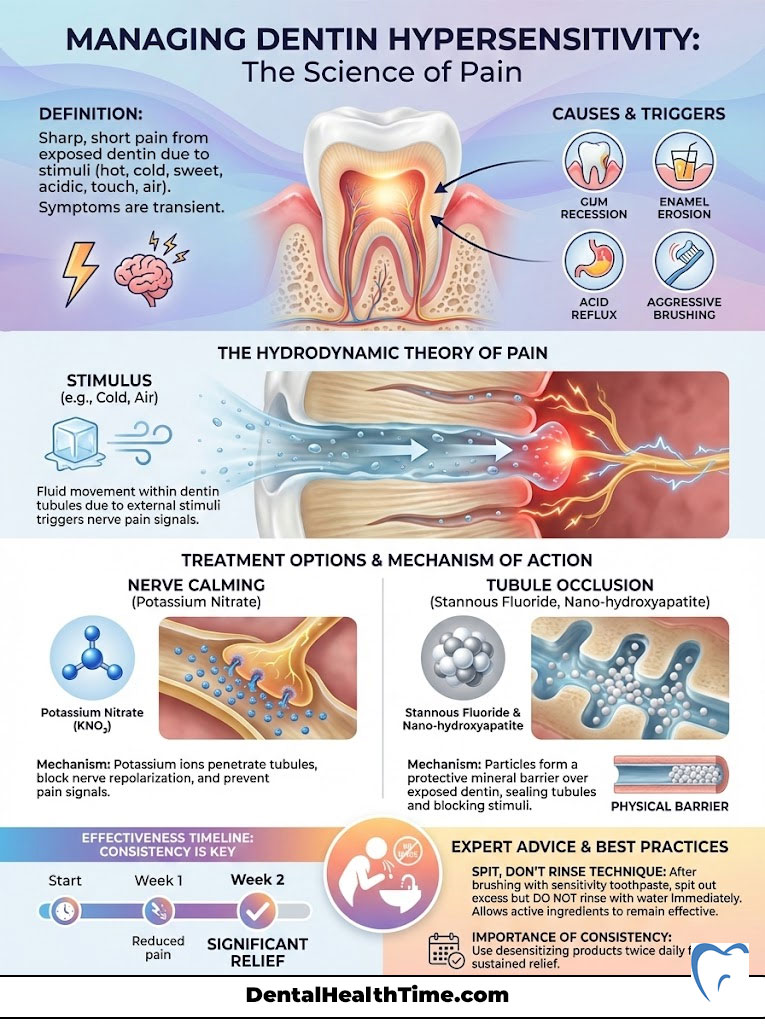
Nerve Calming vs. Tubule Occlusion
To treat this, we have two distinct pharmacological routes. First, we can numb the nerve. Potassium nitrate is the gold standard for this approach. It penetrates the tubule and travels to the pulp. Once there, it depolarizes the nerve ending. This prevents the nerve from firing pain signals to the brain. However, patience is required. Potassium nitrate toothpastes typically take two weeks of twice-daily use to reach full therapeutic efficacy. You cannot use it once and expect a miracle.
The second route is plugging the holes. Stannous fluoride creates a smear layer that physically blocks (occludes) the open tubules. This stops the fluid movement that causes the pain. Nano-hydroxyapatite also excels here. It crystallizes inside the tubules, creating a permanent seal. For severe sensitivity, I often recommend a product that combines stannous fluoride with tubule-occluding technology.
Expert Advice: When using sensitivity toothpaste, do not rinse your mouth with water immediately after brushing. Spit out the excess foam, but leave the residue on your teeth. This is called the “Spit, Don’t Rinse” technique. It gives the potassium nitrate or stannous fluoride more time to penetrate the tooth structure. Rinsing immediately washes away the medicine before it can work.
Gum Health and Gingivitis Control
While most people focus on the white part of the tooth, the pink part is just as important. Gingivitis prevention requires managing the bacteria that cause inflammation. If your gums bleed when you floss, you have an active infection. This is not normal. It is a sign that your oral microbiome is out of balance.
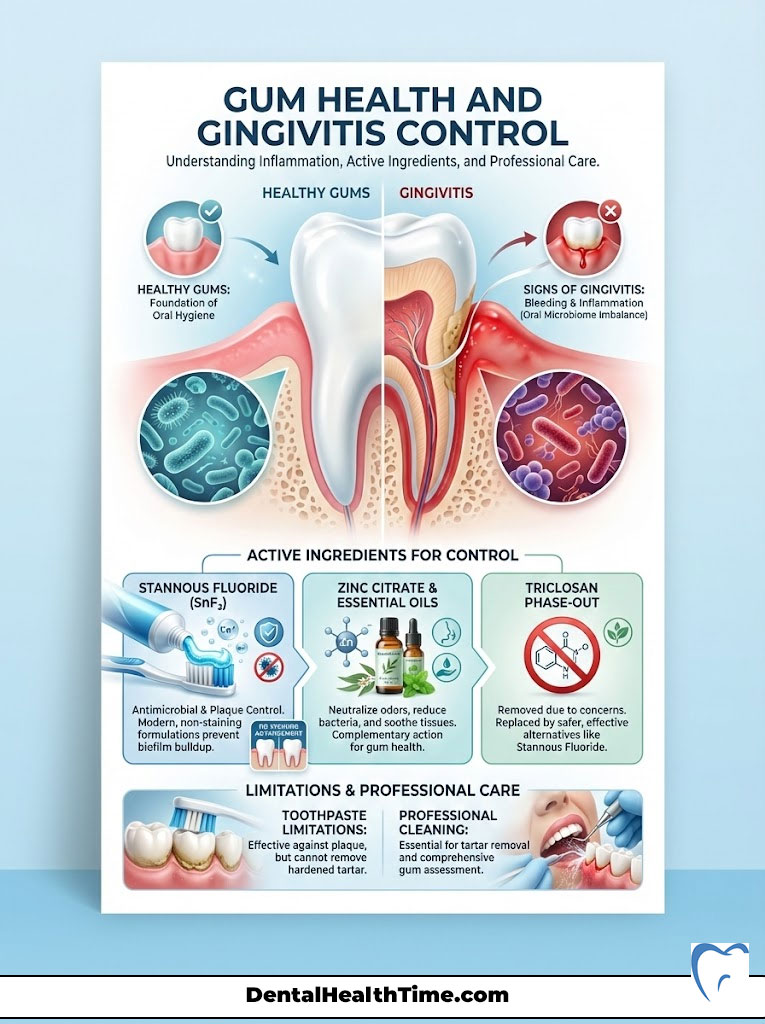
The Role of Stannous Fluoride
Stannous fluoride is a powerhouse for gum health. Unlike sodium fluoride, which strictly hardens enamel, stannous fluoride is broad-spectrum antimicrobial. It kills the bacteria responsible for gum disease and bad breath. It disrupts the metabolic process of the bacteria. In the past, older formulations of stannous fluoride had a reputation for staining teeth yellow. Fortunately, modern chemistry has stabilized the ingredient. Manufacturers now use zinc phosphate or other stabilizers to make staining a non-issue in current products.
The Triclosan Phase-Out
For years, Triclosan was a popular antibacterial agent in toothpaste. It was effective at reducing plaque and gingivitis. However, due to concerns over endocrine disruption and environmental accumulation, it has largely been removed from the market. Today, we rely on Stannous Fluoride and Zinc Citrate to perform the heavy lifting for biofilm control. Essential oils (like those found in Listerine) are also appearing in some paste formulations to aid in tissue health.
Remember, toothpaste is an adjunct. It assists in reducing bacterial load. However, it cannot remove hardened calculus (tartar). Once plaque calcifies into tartar, no toothpaste in the world can remove it. Only a professional hygiene scaling can mechanically fracture that bond.
The Whitening Paradox: Chemical vs. Mechanical
Every patient wants whiter teeth. It is the most requested aesthetic improvement. But there is a fundamental misunderstanding of how whitening toothpaste works. We must distinguish between removing extrinsic stains (coffee, tea, wine) and changing intrinsic tooth color (the natural shade of your dentin).
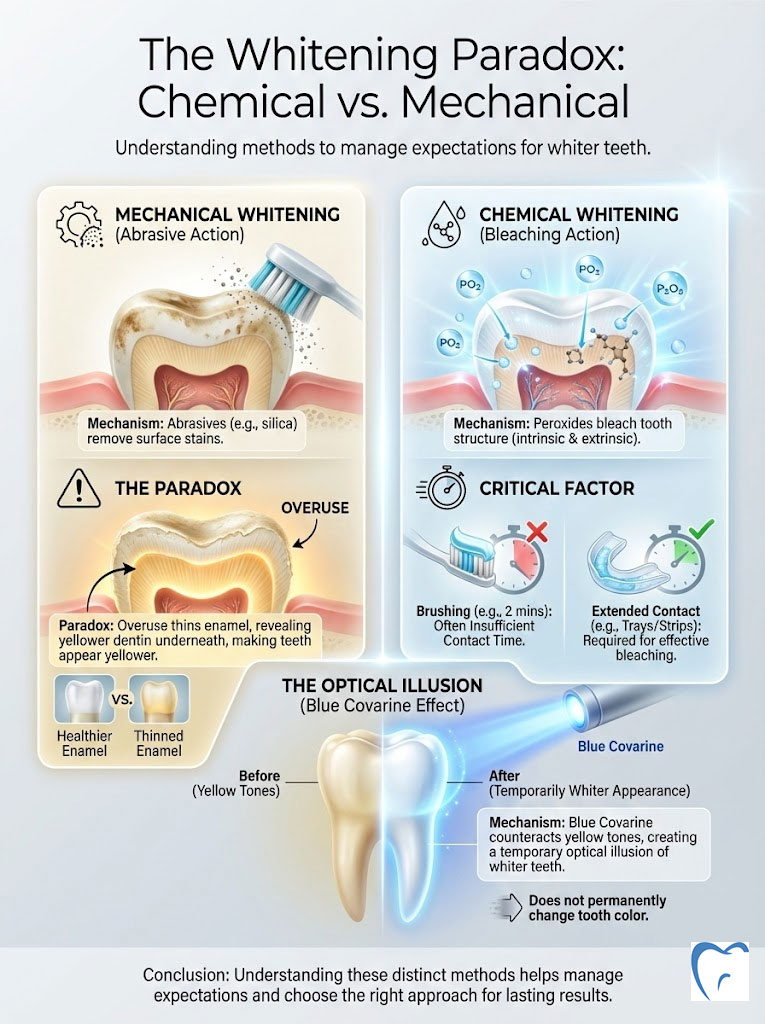
Mechanical Whitening (Abrasives)
Most “whitening” toothpastes rely on abrasives like silica or aluminum oxide. They work by scratching off the surface pigment. Think of it like using a scrub sponge on a counter. While this reveals the cleaner enamel underneath, it does not change the natural shade of your tooth. If you use these high-RDA pastes too aggressively, you will thin the enamel. Since enamel is white and the dentin underneath is yellow, thinning the enamel actually makes your teeth look yellower and more translucent over time. This is the irony of whitening toothpastes.
Chemical Whitening (Peroxides)
To actually bleach the tooth structure, you need hydrogen peroxide or carbamide peroxide. Some high-end toothpastes contain these ingredients. However, the contact time is the limiting factor. Brushing for two minutes is rarely enough time for the peroxide to penetrate the enamel and oxidize deep stains. These products can help maintain a white smile after professional bleaching, but they rarely transform a shade significantly on their own.
Optical Illusions and Blue Covarine
Some brands use a clever trick involving Blue Covarine. This is a blue pigment that deposits on the tooth surface. Blue is opposite yellow on the color wheel. By neutralizing the yellow tones, the tooth appears whiter to the human eye immediately after brushing. It is a temporary optical illusion. It does not change the tooth, but it provides an instant visual boost. This is excellent for a quick fix before an event, but it is not a permanent solution.
Special Considerations: Dry Mouth and Allergies
Sometimes, the ingredients designed to clean your teeth can attack your soft tissues. The oral mucosa is a delicate membrane. It absorbs chemicals rapidly. If you have ever experienced the inside of your cheeks peeling or sloughing off in stringy white pieces, you are likely reacting to the detergent.
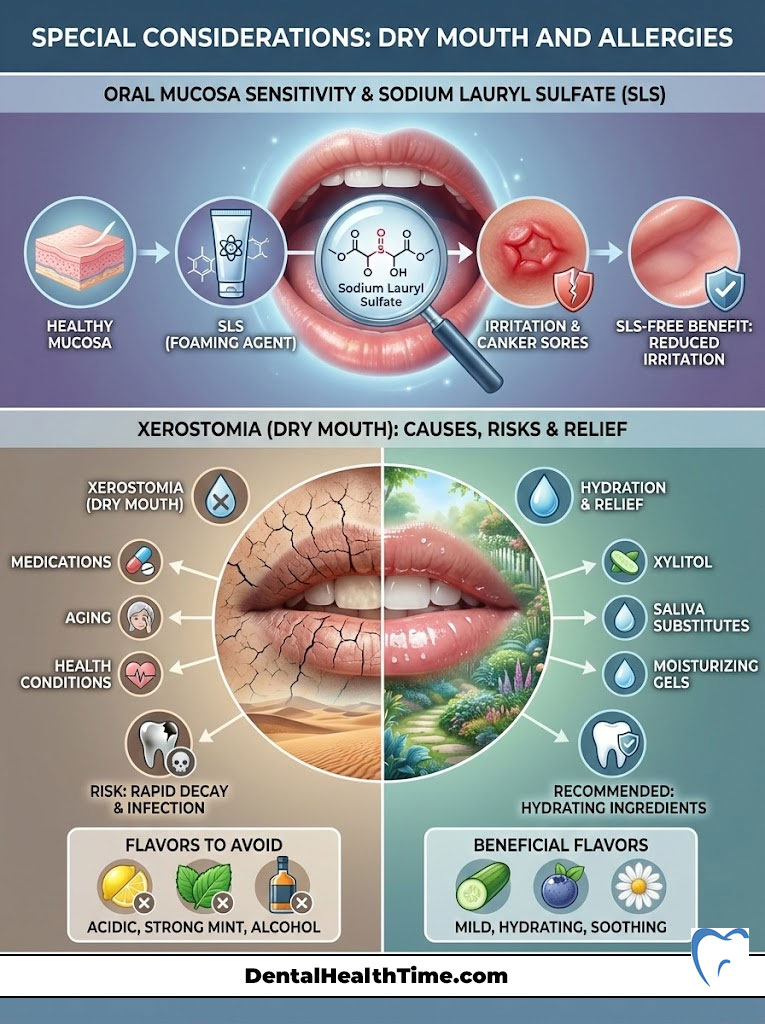
Sodium Lauryl Sulfate (SLS) Sensitivity
Sodium Lauryl Sulfate (SLS) is the industry standard foaming agent. It is cheap and effective. However, it is a harsh detergent. For patients prone to canker sores (aphthous ulcers), SLS can strip the protective mucin layer of the mouth. This leaves the tissue vulnerable to acidic foods and trauma, triggering outbreaks. Switching to a Sodium Lauryl Sulfate (SLS) free toothpaste is often the only change needed to stop chronic canker sores. Look for products that use mild surfactants like sodium lauroyl sarcosinate or cocamidopropyl betaine instead.
Xerostomia (Dry Mouth)
Xerostomia (dry mouth) relief is a major concern. It affects patients on multiple medications, those with autoimmune disorders like Sjogren’s, and those who have undergone radiation therapy. Saliva is your mouth’s natural buffer against acid. It contains calcium and phosphate. Without it, decay progresses rapidly. Patients with dry mouth should avoid strong mint or cinnamon flavors. These oils can sting delicate, dry tissues. Instead, seek products containing enzymes like lactoperoxidase and lysozyme. These mimic the natural proteins in saliva. Also, look for Betaine, which helps retain water in the cells.
The pH Factor: Why Acidity Matters
We rarely talk about the pH of the toothpaste itself. However, it is crucial. The critical pH of enamel is 5.5. Below this level, enamel begins to demineralize (dissolve). Dentin dissolves at an even higher pH of 6.5. Some “natural” or whitening toothpastes can be surprisingly acidic.
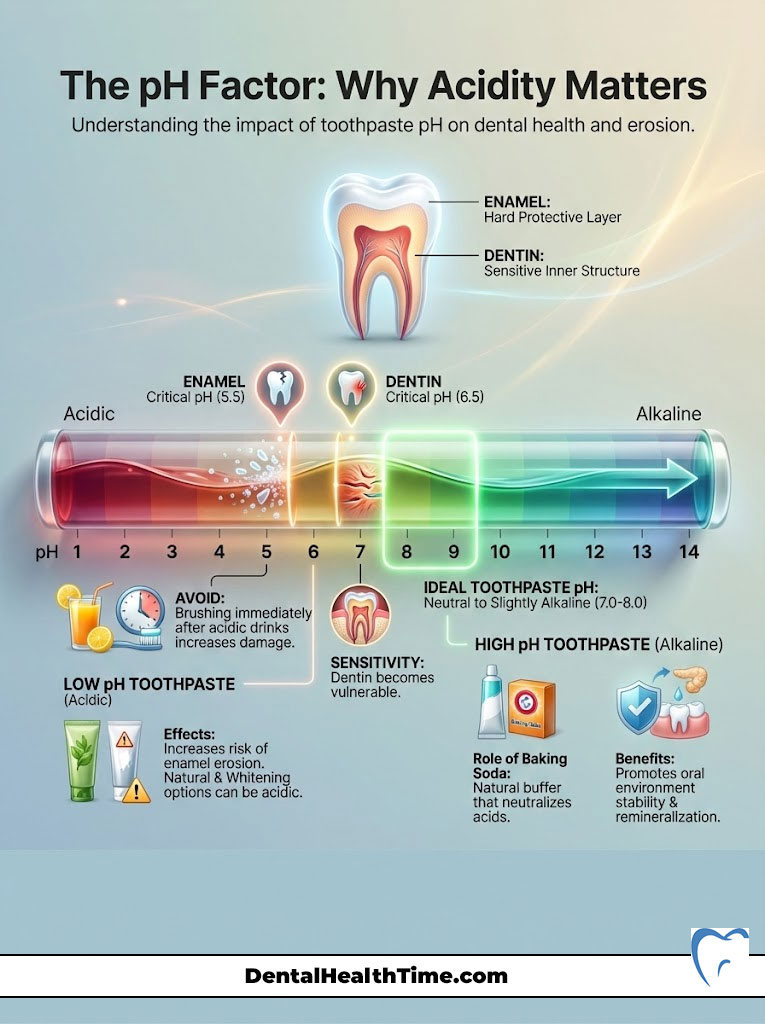
If a toothpaste has a low pH, it can actually contribute to erosion. This is especially true if you brush immediately after drinking acidic soda or juice. You are essentially brushing acid into the teeth. An ideal toothpaste should be neutral or slightly alkaline (pH 7.0 to 8.0). This helps buffer the mouth. It neutralizes the acids produced by bacteria. Baking soda toothpastes are excellent for this. Sodium bicarbonate is a natural buffer. It raises the pH of the oral environment, making it difficult for acid-loving bacteria to survive.
Comparison: Toothpaste Categories and RDA Levels
To help you visualize the safety profile of different products, I have categorized them by their typical abrasivity. This table clarifies which products are safe for the long haul and which should be used sparingly.
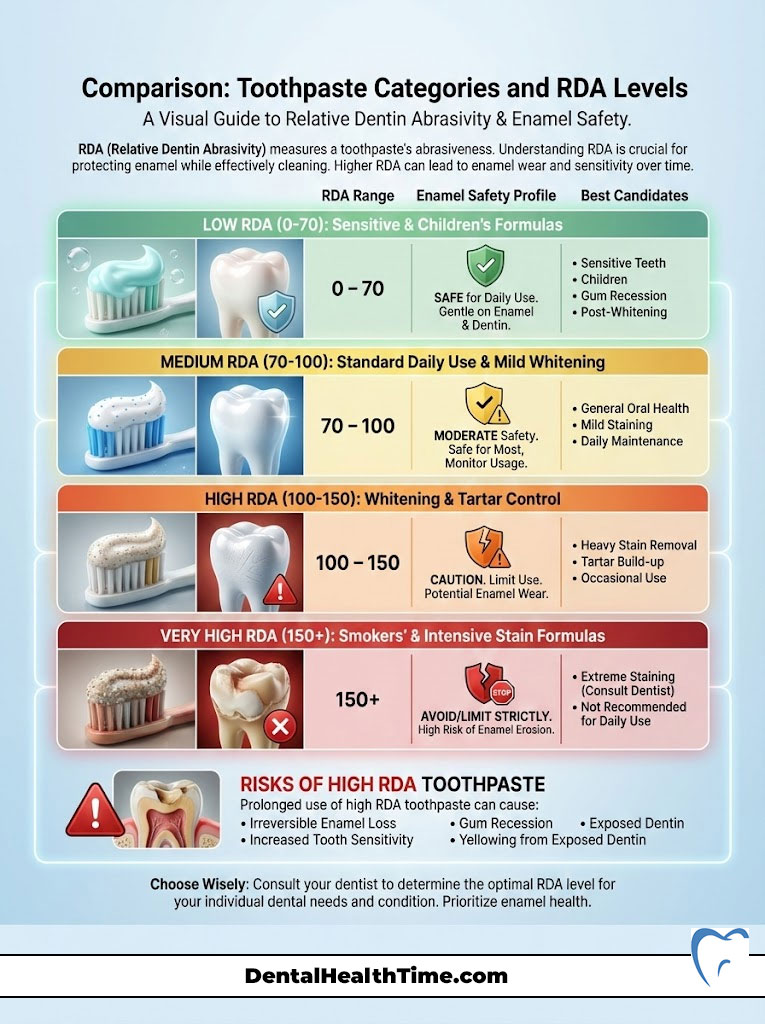
| Toothpaste Category | Typical RDA Range | Enamel Safety Profile | Best Candidate |
|---|---|---|---|
| Plain Baking Soda | 7 (Very Low) | Extremely Safe | Patients with erosion, veneers, or crowns |
| Sensitive Formulas | 30–60 | Very Safe | Exposed roots; post-periodontal surgery |
| Standard Anti-Cavity | 70–100 | Safe for Daily Use | Healthy adults with intact enamel |
| Tartar Control | 90–120 | Moderate Caution | History of heavy calculus buildup |
| “Whitening” Pastes | 100–150 | Caution Required | Thick enamel; heavy coffee/tea drinkers |
| Charcoal / Smokers | 150–200+ | High Risk | Generally not recommended by dentists |
Pediatric Considerations: From First Tooth to Teens
Parents often ask me how to choose the right toothpaste for their children. The balance here is delicate. We must prevent cavities while preventing fluorosis. Fluorosis is a cosmetic defect caused by ingesting too much fluoride while teeth are developing. It appears as white spots or streaks on the adult teeth.

Dosage Guidelines
The American Academy of Pediatric Dentistry (AAPD) recommends using fluoride toothpaste as soon as the first tooth erupts. However, the volume is key. For children under three years old, use a “smear.” This is the size of a grain of rice. It provides the benefit of topical fluoride with minimal systemic risk if swallowed. For children aged three to six, use a pea-sized amount. This ensures that even if they swallow it, the systemic load is safe.
Flavor Fatigue and Compliance
Adults underestimate the “spicy” nature of mint. Menthol triggers the cold receptors in the mouth. For a child, this sensation can feel like burning. Many children refuse to brush simply because the flavor hurts. Do not force adult toothpaste on a child. Fruit, bubblegum, or mild vanilla flavors improve compliance. Compliance is the most important factor in pediatric oral hygiene. As long as the paste contains fluoride, the flavor is irrelevant to the clinical outcome. Find a flavor they love, and they will let you brush longer.
The Orthodontic Challenge
Teens with braces are in a high-risk category. The brackets trap food and plaque. This leads to “white spot lesions” (early decay) around the braces. For orthodontic patients, I often prescribe a high-concentration fluoride paste (5,000 ppm). This requires a prescription. If using over-the-counter products, ensure they use a fluoride rinse in addition to brushing. Nano-hydroxyapatite is also excellent for ortho patients as it helps remineralize lesions without the risk of staining.
The “Natural” Toothpaste Market: Fact vs. Fiction
The natural personal care market is booming. Patients are increasingly wary of synthetic chemicals. This is understandable. However, “natural” does not always mean effective. Essential oils and herbal extracts can be beneficial, but they cannot replace the remineralizing power of fluoride or hydroxyapatite.
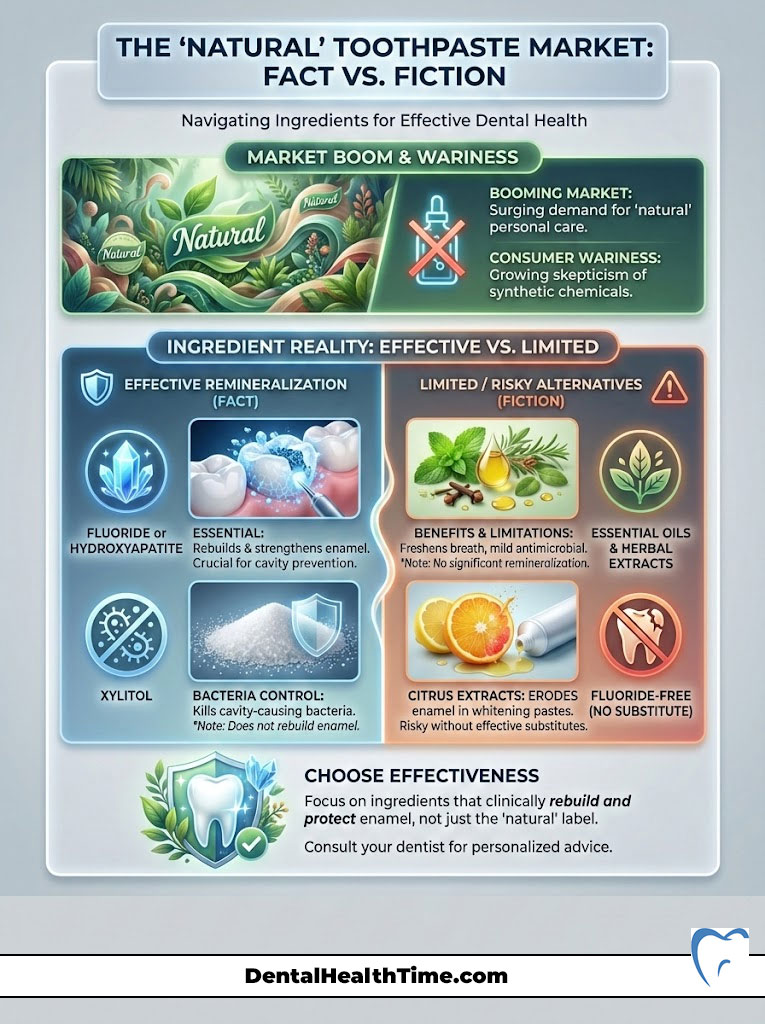
Ingredients That Have Merit
- Tea Tree Oil: Shows genuine promise as an antibacterial and anti-inflammatory agent. It can help reduce gingival bleeding.
- Aloe Vera: Soothing for irritated gums. It is non-abrasive and safe for sensitive tissues.
- Neem: Used in Ayurvedic medicine for centuries. It has antibacterial properties that reduce plaque levels.
- Propolis: A resin produced by bees. It effectively inhibits the bacteria that cause cavities.
Ingredients to Avoid
Be wary of “fluoride-free” pastes that do not substitute it with nano-hydroxyapatite. If you remove the fluoride and replace it with Xylitol alone, you are losing the remineralization benefit. Xylitol kills bacteria, but it does not rebuild enamel. Also, avoid citrus extracts (lemon, lime) in “whitening” natural pastes. Citric acid erodes enamel. Using acid to clean your teeth is counterproductive.
Sustainability: Tube vs. Tablet
We discard billions of toothpaste tubes every year. They are typically made of multi-layer plastics and aluminum, making them nearly impossible to recycle. The industry is shifting. Major brands are moving to HDPE tubes (High-Density Polyethylene), which are technically recyclable. However, the infrastructure to recycle them is not yet widespread.

The Rise of Toothpaste Tablets
Toothpaste tablets (“bits”) are a sustainable alternative. You chew the tablet, mix it with saliva, and brush. They come in glass jars or compostable pouches. From a clinical perspective, tablets are effective if they contain fluoride or n-HAp. Many early tablet brands were fluoride-free, which I could not recommend. Now, many contain adequate fluoride. The main issue is abrasivity. Tablets rely on you chewing them into a fine paste. If you don’t chew them thoroughly, the gritty chunks can be too abrasive. Ensure you chew them completely before applying the brush.
Summary & Key Takeaways
Choosing a toothpaste is not about the prettiest box; it is about matching the chemistry to your biology. We have covered the spectrum from the remineralizing power of Sodium Fluoride vs Stannous Fluoride to the dangers of high-RDA charcoal pastes. Your dental care regimen should be a targeted therapy.
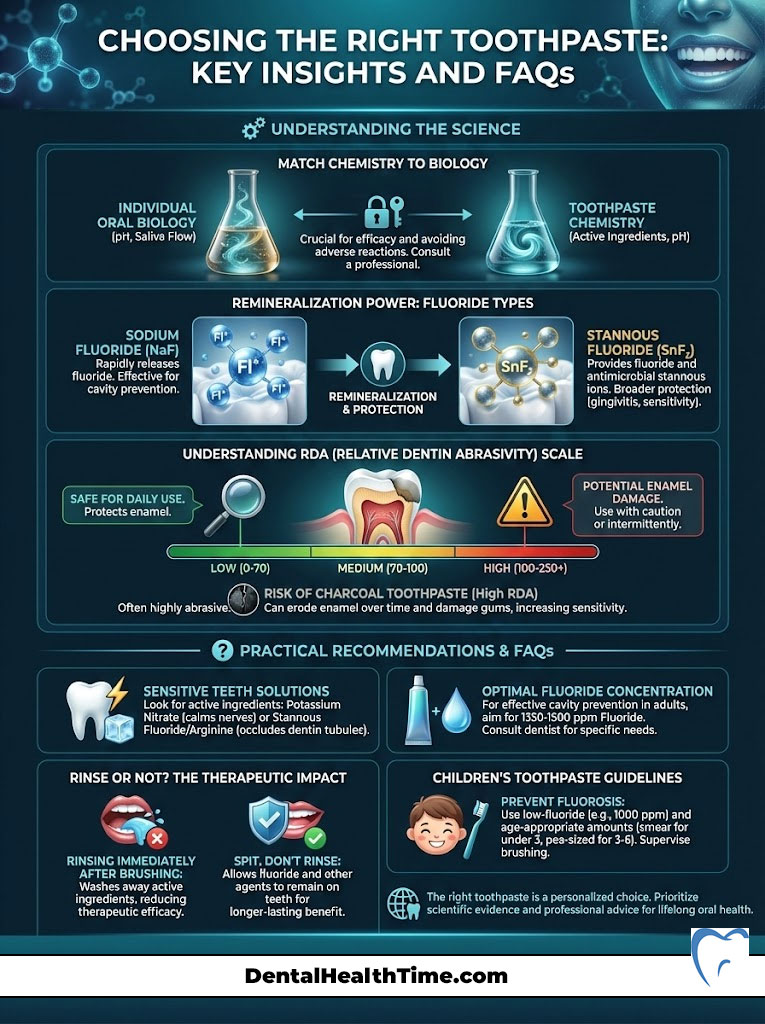
The Final Expert Verdict:
- Check the Seal: The ADA Seal of Acceptance is your baseline for safety. It ensures the product does what it claims.
- Check the Active Ingredient: If you have cavities, get fluoride or n-HAp. If you have bleeding gums, get Stannous Fluoride. If you have pain, get Potassium Nitrate.
- Check the Abrasivity: When in doubt, go low. An RDA under 70 will keep your enamel intact for life. Avoid charcoal if you value your enamel thickness.
- Check the pH: Ensure your product is not acidic. Neutral or alkaline pastes protect against erosion.
- Listen to Your Mouth: If the tissue peels, switch to an SLS-free formula immediately.
Next time you visit your dental hygienist, bring your current tube of toothpaste with you. Ask for a professional audit. We can look at the ingredients together and determine if your choice is helping or hurting your specific case. Your teeth are the only part of your body that cannot heal itself once lost. Choose your tools wisely.
Frequently Asked Questions
What is the Relative Dentin Abrasivity (RDA) scale and why does it matter?
The RDA scale is a standardized metric used to measure the erosive potential of a toothpaste on your dentin and enamel. As a clinician, I recommend selecting a product with an RDA score under 70 for daily use to prevent permanent enamel thinning and tooth wear.
Is nano-hydroxyapatite as effective as fluoride for remineralizing enamel?
Clinical research indicates that high-concentration nano-hydroxyapatite (n-HAp) is a biomimetic alternative that performs comparably to fluoride in preventing caries. While fluoride creates acid-resistant fluorapatite, n-HAp physically fills microscopic lesions and dentin tubules to restore the tooth’s mineral density.
How do I choose the best toothpaste for sensitive teeth?
You should look for active ingredients like potassium nitrate, which depolarizes the nerve to block pain signals, or stannous fluoride, which physically occludes the open dentin tubules. For maximum therapeutic efficacy, avoid high-abrasivity whitening formulas and allow the paste to remain in contact with the teeth for at least two minutes.
Why does my toothpaste cause the skin inside my mouth to peel?
This sloughing of the oral mucosa is often a localized reaction to Sodium Lauryl Sulfate (SLS), a harsh detergent used as a foaming agent. If you experience tissue peeling or chronic canker sores, I advise switching to an SLS-free formulation that utilizes milder surfactants like cocamidopropyl betaine.
Can whitening toothpaste actually change the natural color of my teeth?
Most whitening toothpastes only remove extrinsic surface stains through mechanical abrasives rather than altering the intrinsic shade of your dentin. To truly bleach the internal tooth structure, you require professional-grade peroxides, as the short contact time during brushing is generally insufficient for significant chemical oxidation.
What is the recommended fluoride concentration for adult toothpaste?
For effective cavity prevention and enamel fortification in adults, your toothpaste should contain between 1,000 and 1,500 ppm (parts per million) of fluoride. This concentration ensures that the enamel can undergo proper remineralization and resist the lactic acid produced by the oral microbiome.
Is charcoal toothpaste safe for daily use on tooth enamel?
I generally advise against the daily use of charcoal toothpaste because many formulations have a dangerously high RDA score that acts like fine sandpaper. While they effectively scrub away surface stains, they can permanently wear down your enamel, eventually making teeth appear yellower as the underlying dentin is exposed.
How much toothpaste should I use for my child to avoid fluorosis?
To prevent dental fluorosis, children under age three should only use a \”smear\” or grain-of-rice-sized amount of fluoride toothpaste. For children aged three to six, a pea-sized amount is the clinical standard to provide adequate topical protection while minimizing the risk of systemic ingestion.
What is the benefit of stannous fluoride over sodium fluoride?
While sodium fluoride is excellent for hardening enamel, stannous fluoride is a multi-tasking agent with broad-spectrum antimicrobial properties. It effectively kills the bacteria responsible for gingivitis and halitosis while also creating a smear layer to block sensitivity triggers in the dentin tubules.
Why should I stop rinsing my mouth with water after brushing?
Rinsing immediately after brushing washes away the active therapeutic ingredients before they can chemically bond with your enamel. By using the \”spit, don’t rinse\” technique, you maximize the contact time for fluoride or nano-hydroxyapatite to penetrate the tooth structure and provide lasting protection.
Can toothpaste tablets effectively prevent cavities?
Toothpaste tablets can be effective if they contain a remineralizing agent like fluoride or nano-hydroxyapatite and are used correctly. You must ensure you chew them into a fine, smooth paste before brushing to avoid uneven abrasivity and to ensure the active ingredients are properly distributed across all tooth surfaces.
What role does pH play in choosing a toothpaste for acid erosion?
Enamel begins to dissolve at a critical pH of 5.5, so your toothpaste should ideally be neutral or slightly alkaline (pH 7.0 to 8.0) to buffer oral acids. Formulations containing sodium bicarbonate (baking soda) are particularly effective at raising the pH of the oral environment, making it difficult for acid-loving, cariogenic bacteria to survive.
Disclaimer
This article is for informational and educational purposes only and does not constitute professional medical or dental advice, diagnosis, or treatment. Always seek the advice of your dentist, dental hygienist, or other qualified healthcare provider with any questions you may have regarding an oral health condition or the selection of dental products. Never disregard professional medical advice or delay in seeking it because of something you have read in this article.
References
Provide 5-8 authoritative reference sources that support the article content:
- American Dental Association (ADA) – Seal of Acceptance Program – Standards for toothpaste safety, efficacy, and fluoride requirements.
- Food and Drug Administration (FDA) – 21 CFR Part 355 – Regulations regarding anticaries drug products for over-the-counter human use.
- International Organization for Standardization (ISO) – ISO 11609:2017 – Dentistry — Dentifrices — Requirements, test methods and marking for abrasivity.
- American Academy of Pediatric Dentistry (AAPD) – Fluoride Therapy Guidelines – Official recommendations for fluoride use in children and preventing fluorosis.
- Journal of Clinical Dentistry – Relative Dentin Abrasivity (RDA) Studies – Peer-reviewed research on the impact of abrasive dentifrices on tooth structure.
- Sjogren’s Foundation – Xerostomia Patient Education – Clinical insights into managing dry mouth and selecting non-irritating oral care products.


1 thought on “How to Choose the Right Toothpaste for Your Dental Needs?”